Loss of biochemical response at any time worsens outcomes in UDCA-treated patients with primary biliary cholangitis
- PMID: 39380718
- PMCID: PMC11460452
- DOI: 10.1016/j.jhepr.2024.101168
Loss of biochemical response at any time worsens outcomes in UDCA-treated patients with primary biliary cholangitis
Erratum in
-
Erratum regarding previously published articles.JHEP Rep. 2025 Feb 17;7(3):101359. doi: 10.1016/j.jhepr.2025.101359. eCollection 2025 Mar. JHEP Rep. 2025. PMID: 40170909 Free PMC article.
Abstract
Background & aims: Biochemical response to ursodeoxycholic acid (UDCA) therapy is associated with good prognosis in people living with primary biliary cholangitis (PBC). Biochemical response is typically assessed early in disease and it is not known what proportion of patients lose previously attained biochemical response, nor whether this impacts long-term liver transplant (LT)-free survival.
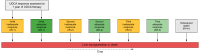
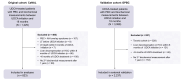
Methods: We identified all UDCA-treated patients with PBC from the Canadian Network for Autoimmune Liver disease with biochemical measurements at 1 year, and evaluated their liver biochemistry over time. Inadequate biochemical response was defined as serum alkaline phosphatase ≥1.67x the upper limit of normal or abnormal serum total bilirubin at 1 year of UDCA therapy and all time points thereafter. Multistate Markov models were used to estimate transition rates between biochemical response states and from each state to LT or death. Results were validated in an external cohort (GLOBAL PBC registry).
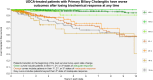
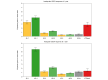
Results: A total of 823 patients from eight centers were included. Mean age at diagnosis was 53 years, 91% were female, 33% had inadequate biochemical response to UDCA at 1 year (n = 269). Patients who retained initial adequate response had lower rates of LT or death compared to patients who subsequently lost response (relative rate 0.102, 95% CI 0.047-0.223). Patients who regained adequate response had lower rates than patients who did not (0.016, 95% CI 0.001-0.568), and patients who lost response once more (0.010, 95% CI 0.001-0.340). Patients who regained adequate response for a third time also had lower rates than patients who did not (0.151, 95% CI 0.040-0.566). Analyses in the GLOBAL PBC registry (n = 2,237) validated these results.
Conclusion: Loss of biochemical response at any time is associated with heightened risks of LT or death in people living with PBC. Achievement of biochemical response is an important goal throughout follow-up, regardless of biochemical response profile early in therapy.
Impact and implications: Early biochemical response to ursodeoxycholic acid is associated with good prognosis in patients with primary biliary cholangitis (PBC). Our work demonstrates that patients with PBC transition between biochemical response states over time, and that these transitions correspond with changes in risk of liver transplantation or death. Clinicians should re-evaluate risk and optimize treatment decisions for patients with PBC throughout follow-up, regardless of early biochemical response to therapy.
Keywords: UDCA; alkaline phosphatase; liver transplantation; prognostication; total bilirubin.
© 2024 The Author(s).
Figures