Combined inhibition of CTPS1 and ATR is a metabolic vulnerability in p53-deficient myeloma cells
- PMID: 39380841
- PMCID: PMC11460984
- DOI: 10.1002/hem3.70016
Combined inhibition of CTPS1 and ATR is a metabolic vulnerability in p53-deficient myeloma cells
Abstract
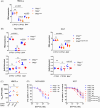
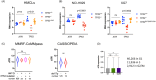
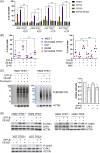
In multiple myeloma, as in B-cell malignancies, mono- and especially bi-allelic TP53 gene inactivation is a high-risk factor for treatment resistance, and there are currently no therapies specifically targeting p53 deficiency. In this study, we evaluated if the loss of cell cycle control in p53-deficient myeloma cells would confer a metabolically actionable vulnerability. We show that CTP synthase 1 (CTPS1), which encodes a CTP synthesis rate-limiting enzyme essential for DNA and RNA synthesis in lymphoid cells, is overexpressed in samples from myeloma patients displaying a high proliferation rate (high MKI67 expression) or a low p53 score (synonymous with TP53 deletion and/or mutation). This overexpression of CTPS1 was associated with reduced survival in two cohorts. Using scRNA-seq analysis in 24 patient samples, we further demonstrate that myeloma cells in the S or G2/M phase display high CTPS1 expression. Pharmacological inhibition of CTPS1 by STP-B induced cell cycle arrest in early S phase in isogenic NCI-H929 or XG7 TP53 +/+, TP53 -/-, and TP53 R175H/R175H cells and in a TP53 -/R123STOP patient sample. The functional annotation of transcriptional changes in 10 STP-B-treated myeloma cell lines revealed a decrease in protein translation and confirmed the blockade of cells into the S phase. The pharmacological inhibition of ATR, which governs the intrinsic S/G2 checkpoint, in STP-B-induced S-phase arrested cells synergistically induced cell death in TP53 +/+, TP53 -/-, and TP53 R175H/R175H isogenic cell lines (Bliss score >15). This combination induced replicative stress and caspase-mediated cell death and was highly effective in resistant/refractory patient samples with TP53 deletion and/or mutation and in TP53 -/- NCI-H929 xenografted NOD-scid IL2Rgamma mice. Our in vitro, ex vivo, and in vivo data provide the rationale for combined CTPS1 and ATR inhibition for the treatment of p53-deficient patients.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Hélène Asnagli, Andrew Parker, and Philip Beer are employees of Step Pharma. The remaining authors declare no conflict of interest.
Figures





References
-
- Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem‐cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open‐label, phase 3 study. Lancet. 2019;394(10192):29‐38. - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B‐cell maturation antigen‐directed chimeric antigen receptor T‐cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE‐1): a phase 1b/2 open‐label study. Lancet. 2021;398(10297):314‐324. - PubMed
-
- Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705‐716. - PubMed
-
- Manier S, Ingegnere T, Escure G, et al. Current state and next‐generation CAR‐T cells in multiple myeloma. Blood Rev. 2022;54:100929. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
