Safe and potent anti-CD19 CAR T-cells with shRNA-IL-6 gene silencing element in patients with refractory or relapsed B-cell acute lymphoblastic leukemia
- PMID: 39380843
- PMCID: PMC11456753
- DOI: 10.1002/hem3.70007
Safe and potent anti-CD19 CAR T-cells with shRNA-IL-6 gene silencing element in patients with refractory or relapsed B-cell acute lymphoblastic leukemia
Abstract
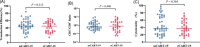
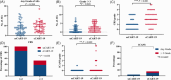
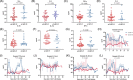
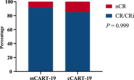
Severe cytokine release syndrome (sCRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) have limited the widespread use of chimeric antigen receptor T (CAR T)-cell therapy. We designed a novel anti-CD19 CAR (ssCART-19) with a small hairpin RNA (shRNA) element to silence the interleukin-6 (IL-6) gene, hypothesizing it could reduce sCRS and ICANS by alleviating monocyte activation and proinflammatory cytokine release. In a post hoc analysis of two clinical trials, we compared ssCART-19 with common CAR T-cells (cCART-19) in relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL). Among 87 patients, 47 received ssCART-19 and 40 received cCART-19. Grade ≥3 CRS occurred in 14.89% (7/47) of the ssCART-19 group versus 37.5% (15/40) in the cCART-19 group (p = 0.036). ICANS occurred in 4.26% (2/47) of the ssCART-19 group (all grade 1) compared to 15% (2/40) of the cCART-19 group. Patients in the ssCART-19 group showed comparable rates of treatment response (calculated with rates of complete remission and incomplete hematological recovery) were 91.49% (43/47) for ssCART-19 and 85% (34/40) for cCART-19 (p = 0.999). With a median follow-up of 21.9 months, cumulative nonrelapse mortality was 10.4% for ssCART-19 and 13.6% for cCART-19 (p = 0.33). Median overall survival was 37.17 months for ssCART-19 and 32.93 months for cCART-19 (p = 0.40). Median progression-free survival was 24.17 months for ssCART-19 and 9.33 months for cCART-19 (p = 0.23). These data support the safety and efficacy of ssCART-19 for r/r B-ALL, suggesting its potential as a promising therapy.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

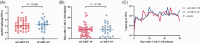
References
LinkOut - more resources
Full Text Sources
