Outcomes and prognostic factors in 3306 patients with relapsed/refractory chronic lymphocytic leukemia treated with ibrutinib outside of clinical trials: A nationwide study
- PMID: 39380844
- PMCID: PMC11459203
- DOI: 10.1002/hem3.70017
Outcomes and prognostic factors in 3306 patients with relapsed/refractory chronic lymphocytic leukemia treated with ibrutinib outside of clinical trials: A nationwide study
Abstract
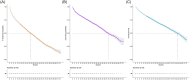
We performed a cohort study that included all patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) who received ibrutinib in the Italian National Health Service. With a median follow-up of 42.2 months (IQR 30.8-54.6 months), the study involved 3306 patients with a median age of 72.1 years, of whom 42.6% had received ≥2 previous lines of treatment. The estimated 24-month probabilities of being on treatment and alive were 57.9% (95% confidence interval [CI]: 59.6-56.2) and 76.6% (95% CI: 75.2-78.1), respectively. The median time to treatment discontinuation (TTD) was 31.3 months (95% CI: 29.5-33.5). Out of 3306 patients, 2015 (60.9%) discontinued treatment, with 993 cases attributed to death or disease progression (30.0% of all cases). Among the 1022 patients who discontinued treatment for reasons other than progression or death, 564 (17.1%) patients did so due to toxicity or medical decision, while 458 patients (13.8%) were lost to follow-up. Multivariable analysis revealed that age, Eastern Cooperative Oncology Group Performance Status, the number of previous lines of therapy, refractoriness to the last treatment, and reduced renal function were associated with shorter TTD and overall survival (OS). The coexistence of 17p- and TP53 mutations had an independent unfavorable impact on TTD and OS. Nonstandard doses were associated with shorter TTD and advanced stage with shorter OS. The median OS postprogression and postdiscontinuation for other reasons were estimated at 12.9 (95% CI: 11.3-16.2) and 22.7 months (95% CI: 20.2-28.3), respectively. This large real-world study shows that ibrutinib is an effective treatment for R/R CLL. Baseline patient characteristics and double-hit TP53 aberrations were associated with inferior prognosis, and discontinuation due to CLL progression portended a poor outcome.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Gian Matteo Rigolin received honoraria for participation in the speaker's bureau from Abbvie, AstraZeneca, and Janssen, as well as travel grants from Janssen. Lydia Scarfò received honoraria for advisory board participation from AbbVie, AstraZeneca, BeiGene, and Janssen, as well as travel grants from Beigene and Janssen; she is on the speaker bureau for Octapharma. Antonio Cuneo received honoraria for participation in the speaker's bureau and advisory board from Abbvie, AstraZeneca, Beigene, Janssen, and Lilly. Paolo Ghia received research support from AbbVie, AstraZeneca, BMS, and Janssen and honoraria from AbbVie, AstraZeneca, BeiGene, BMS, Janssen, Lilly/Loxo Oncology, MSD, and Roche, and is an editor of HemaSphere. The remaining authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous