A disproportionality analysis for assessing the safety of FLT3 inhibitors using the FDA Adverse Event Reporting System (FAERS)
- PMID: 39381060
- PMCID: PMC11459563
- DOI: 10.1177/20420986241284105
A disproportionality analysis for assessing the safety of FLT3 inhibitors using the FDA Adverse Event Reporting System (FAERS)
Abstract
Objectives: This pharmacovigilance analysis was conducted to assess the safety signals of FMS-related tyrosine kinase 3 (FLT3) inhibitors in a real-world setting using the United States Food and Drug Administration Adverse Event Reporting System (FAERS).
Design: We analyzed adverse event (AE) reports related to FLT3 inhibitors submitted to the FAERS database from the first quarter of 2015 to the fourth quarter of 2022. Disproportionality analysis was used to identify AEs of FLT3 inhibitors in the FAERS database.
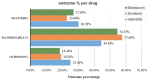
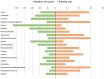
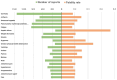
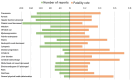
Results: A total of 55,393 AE reports were identified, of which 5938, 44,013, and 5442 were attributed to midostaurin, sorafenib, and gilteritinib, respectively, as primary suspects. Compared to the full database, significant safety signals at the system organ class level were observed for midostaurin (blood and lymphatic system disorders and hepatobiliary disorders), sorafenib (skin and subcutaneous tissue disorders and hepatobiliary disorders), and gilteritinib (investigations, blood and lymphatic system disorders, infections and infestations, and hepatobiliary disorders). All the drugs studied were associated with hepatobiliary disorders. The most prominent AEs associated with midostaurin, sorafenib, and gilteritinib were cytopenia, palmar-plantar erythrodysesthesia syndrome, and increased blast cell count, respectively. Compared with chemotherapy, midostaurin and gilteritinib showed a higher risk of electrocardiogram QT prolongation, gastrointestinal hemorrhage, cerebral hemorrhage, and increased white blood cell count. Gilteritinib had the highest overall death percentage (30.28%), whereas sorafenib had the lowest (23.06%).
Conclusion: Mining AE signals using the FAERS database provides a method for analyzing the safety of FLT3 inhibitors in post-marketing. We found several significant AE signals that corresponded to previous studies; however, some AE signals were not mentioned in the drug instructions. Our study could provide a direction for follow-up real-world studies to verify the results further.
Keywords: FAERS; FLT3 inhibitors; drug safety; gilteritinib; midostaurin; sorafenib.
Plain language summary
A study on the adverse effects of FLT3 inhibitors.
Introduction: The United States Food and Drug Administration Adverse Event Reporting System (FAERS) is an essential tool for the United States Food and Drug Administration (FDA) to detect adverse events (AE). This study explored the safety signals of FMS-related tyrosine kinase 3 (FLT3) inhibitors (midostaurin, sorafenib, and gilteritinib) using the FAERS database.
Research design and methods: We used reporting odds ratios, proportional reporting ratios, and Bayesian confidence propagation neural network to analyze the safety signals of FLT3 inhibitors by comparing them with the full database and chemotherapy agents from 2015 to 2022.
Results: A total of 5,938, 44,013, and 5,442 reports were attributed to midostaurin, sorafenib, and gilteritinib, respectively. Based on the analysis results, we observed the following:• Regarding the analysis of system organ class level compared with the full database, “hepatobiliary disorders” appeared as an important signal in all three drugs. In addition, “blood and lymphatic system disorders” of midostaurin, “skin and subcutaneous tissue disorders” of sorafenib, and “investigations,” “blood and lymphatic system disorders,” and “infections and infestations” of gilteritinib were significant.• Cytopenia was the most prominent AE associated with midostaurin in comparisons of midostaurin versus the full database, and electrocardiogram QT prolonged was the strongest signal in comparisons of midostaurin versus chemotherapy.• In both comparisons of FLT3 inhibitors versus the full database and chemotherapy, the strongest safety signals of sorafenib and gilteritinib were palmar-plantar erythrodysesthesia syndrome and increased blast cell count, respectively.• Gilteritinib exhibited the highest overall mortality rate, whereas sorafenib had the lowest.
Conclusion: We identified several significant AE signals that corresponded to previous studies. However, some AE signals were not mentioned in the drug instructions. The AE signals should be evaluated further based on real-world data in the future.
© The Author(s), 2024.
Figures
Similar articles
-
Unveiling unexpected adverse events: post-marketing safety surveillance of gilteritinib and midostaurin from the FDA Adverse Event Reporting database.Ther Adv Drug Saf. 2025 Jan 10;16:20420986241308089. doi: 10.1177/20420986241308089. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 39802043 Free PMC article.
-
Adverse event profiles of CDK4/6 inhibitors: data mining and disproportionality analysis of the FDA adverse event reporting system.Ther Adv Drug Saf. 2024 Sep 24;15:20420986241278498. doi: 10.1177/20420986241278498. eCollection 2024. Ther Adv Drug Saf. 2024. PMID: 39376495 Free PMC article.
-
A post-marketing disproportionality analysis of the safety of ribociclib based on the FDA Adverse Event Reporting System.Ther Adv Drug Saf. 2025 Feb 28;16:20420986251324633. doi: 10.1177/20420986251324633. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40026915 Free PMC article.
-
FLT3 inhibitors in acute myeloid leukaemia: assessment of clinical effectiveness, adverse events and future research-a systematic review and meta-analysis.Syst Rev. 2020 Dec 7;9(1):285. doi: 10.1186/s13643-020-01540-1. Syst Rev. 2020. PMID: 33287892 Free PMC article.
-
Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors.Drug Saf. 2018 Apr;41(4):357-361. doi: 10.1007/s40264-017-0622-2. Drug Saf. 2018. PMID: 29196988 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

