NLRP3 inflammasome activation and pyroptosis are dispensable for tau pathology
- PMID: 39381137
- PMCID: PMC11458539
- DOI: 10.3389/fnagi.2024.1459134
NLRP3 inflammasome activation and pyroptosis are dispensable for tau pathology
Abstract
Background: Neuroinflammation is widely recognized as a key factor in the pathogenesis of Alzheimer's disease (AD), alongside ß-amyloid deposition and the formation of neurofibrillary tangles. The NLR family pyrin domain containing 3 (NLRP3) inflammasome, part of the innate immune system, has been implicated in the neuropathology of both preclinical amyloid and tau transgenic models. Activation of the NLRP3 pathway involves an initial priming step, which increases the expression of Nlrp3 and interleukin (IL)-1β, followed by the assembly of the NLRP3 inflammasome complex, comprising NLRP3, ASC, and caspase-1. This assembly leads to the proteolytic maturation of the pro-inflammatory cytokines IL-1β and IL-18. Additionally, the NLRP3 inflammasome induces Gasdermin D (GSDMD) cleavage, forming membrane pores through which IL-1β and IL-18 are secreted. Inhibition of NLRP3 has been shown to enhance plaque clearance by modulating microglial activation. Furthermore, blocking NLRP3 in tau transgenic mice has been found to reduce tau phosphorylation by affecting the activity of certain tau kinases and phosphatases.
Methods: In this study, organotypic brain slice cultures from P301S transgenic mice were treated with lipopolysaccharide (LPS) plus nigericin as a positive control or exposed to tau seeds (K18) to evaluate NLRP3 inflammasome activation. The effect of tau seeding on NLRP3 activity was further examined using Meso Scale Discovery (MSD) assays to measure IL1β secretion levels in the presence and absence of NLRP3 inhibitors. The role of NLRP3 activity was investigated in full-body Nlrp3 knockout mice crossbred with the tau transgenic P301S model. Additionally, full-body and microglia-selective Gsdmd knockout mice were crossbred with P301S mice, and tau pathology and neurodegeneration were evaluated at early and late stages of the disease using immunohistochemistry and biochemical assays.
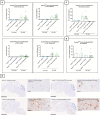
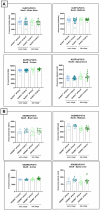
Results: Activation of the NLRP3 pathway was observed in the mouse organotypic slice culture (OSC) model following stimulation with LPS and nigericin or exposure to tau seeds. However, Nlrp3 deficiency did not mitigate tauopathy or neurodegeneration in P301S mice in vivo, showing only a minor effect on plasma neurofilament (NF-L) levels. Consistently, Gsdmd deficiency did not alter tau pathology in P301S mice. Furthermore, neither full-body nor microglia-selective Gsdmd deletion had an impact on neuronal pathology or the release of pro-inflammatory cytokines.
Conclusion: The absence of key components of the NLRP3 inflammasome pathway did not yield a beneficial effect on tau pathology or neurodegeneration in the preclinical Tau-P301S mouse model of AD. Nonetheless, organotypic slice cultures could serve as a valuable ex vivo mechanistic model for evaluating NLRP3 pathway activation and pharmacological inhibitors.
Keywords: GSDMD; NLRP3; OSCs; P301S; neurodegeneration and tau pathology.
Copyright © 2024 Paesmans, Van Kolen, Vandermeeren, Shih, Wuyts, Boone, Garcia Sanchez, Grauwen, Van Hauwermeiren, Van Opdenbosch, Lamkanfi, van Loo and Bottelbergs.
Conflict of interest statement
AB, DW, IP, KG, KV, and NO are employees of Jansssssen Pharmaceutica. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

