Personalized PDAC chip with functional endothelial barrier for tumour biomarker detection: A platform for precision medicine applications
- PMID: 39381267
- PMCID: PMC11460472
- DOI: 10.1016/j.mtbio.2024.101262
Personalized PDAC chip with functional endothelial barrier for tumour biomarker detection: A platform for precision medicine applications
Abstract
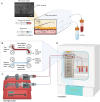
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer characterised by poor survival rates and an increasing global incidence. Advances in the staging and categorization of pancreatic tumours, along with the discovery of functional mutations, have made precision treatments possible, which may lead to better clinical results. To further improve customized treatment approaches, in vitro models that can be used for functional drug sensitivity testing and precisely mimic the disease at the organ level are required. In this study, we present a workflow for creating a personalized PDAC chip utilising primary tumour-derived human pancreatic organoids (hPOs) and Human Umbilical Vein Endothelial Cells (HUVECs) to simulate the vascular barrier and tumour interactions within a PDMS-free organ-on-a-chip system. The patient PDAC tissue, expanded as tumour hPOs, could be cultured as adherent cells on the chip for more than 50 days, allowing continuous monitoring of cell viability through outflows from tumour and endothelial channels. Our findings demonstrate a gradual increase in cell density and cell turnover in the pancreatic tumor channel. Tumour-specific biomarkers, including CA-19.9, TIMP-1, Osteopontin, MIC-1, ICAM-1 and sAXL were consistently detected in the PDAC chip outflows. Comparative analyses between tissue culture plates and microfluidic conditions revealed significant differences in biomarker secretion patterns, highlighting the advantages of the microfluidics approach. This PDAC chip provides a stable, reproducible tumour model system with a functional endothelial cell barrier, suitable for drug sensitivity and secretory biomarker studies, thus serving as a platform for functional precision medicine application and multi-organ chip development.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Rahn S., Zimmermann V., Viol F., Knaack H., Stemmer K., Peters L., et al. Diabetes as risk factor for pancreatic cancer: hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018;415:129–150. doi: 10.1016/j.canlet.2017.12.004. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

