Exploring the role of mitochondrial uncoupling protein 4 in brain metabolism: implications for Alzheimer's disease
- PMID: 39381683
- PMCID: PMC11459774
- DOI: 10.3389/fnins.2024.1483708
Exploring the role of mitochondrial uncoupling protein 4 in brain metabolism: implications for Alzheimer's disease
Abstract
The brain's high demand for energy necessitates tightly regulated metabolic pathways to sustain physiological activity. Glucose, the primary energy substrate, undergoes complex metabolic transformations, with mitochondria playing a central role in ATP production via oxidative phosphorylation. Dysregulation of this metabolic interplay is implicated in Alzheimer's disease (AD), where compromised glucose metabolism, oxidative stress, and mitochondrial dysfunction contribute to disease progression. This review explores the intricate bioenergetic crosstalk between astrocytes and neurons, highlighting the function of mitochondrial uncoupling proteins (UCPs), particularly UCP4, as important regulators of brain metabolism and neuronal function. Predominantly expressed in the brain, UCP4 reduces the membrane potential in the inner mitochondrial membrane, thereby potentially decreasing the generation of reactive oxygen species. Furthermore, UCP4 mitigates mitochondrial calcium overload and sustains cellular ATP levels through a metabolic shift from mitochondrial respiration to glycolysis. Interestingly, the levels of the neuronal UCPs, UCP2, 4 and 5 are significantly reduced in AD brain tissue and a specific UCP4 variant has been associated to an increased risk of developing AD. Few studies modulating the expression of UCP4 in astrocytes or neurons have highlighted protective effects against neurodegeneration and aging, suggesting that pharmacological strategies aimed at activating UCPs, such as protonophoric uncouplers, hold promise for therapeutic interventions in AD and other neurodegenerative diseases. Despite significant advances, our understanding of UCPs in brain metabolism remains in its early stages, emphasizing the need for further research to unravel their biological functions in the brain and their therapeutic potential.
Keywords: Alzheimer’s disease; UCP4; astrocytes; mitochondria; neurons; uncoupling agent; uncoupling protein.
Copyright © 2024 Crivelli, Gaifullina and Chatton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
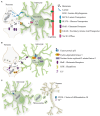

 > 80%,
> 80%,  >60%,
>60%,  > 40%,
> 40%,  < 40%). Residues which have been implicated by site-directed mutagenesis in transport of H+, sensing pH shifts, transport of Cl− or interacting with nucleotide are highlighted across sequences.
< 40%). Residues which have been implicated by site-directed mutagenesis in transport of H+, sensing pH shifts, transport of Cl− or interacting with nucleotide are highlighted across sequences.
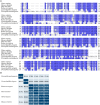
 > 80%,
> 80%,  >60%,
>60%,  > 40%,
> 40%,  < 40%). (B) The identity matrix between UCP4 sequences was generated using UniProt.
< 40%). (B) The identity matrix between UCP4 sequences was generated using UniProt.
References
Publication types
LinkOut - more resources
Full Text Sources

