Platelet factors ameliorate thoracic aortic aneurysm and dissection by inhibiting the FGF-FGFR cascade activation in aortic-endothelial cell
- PMID: 39381736
- PMCID: PMC11460509
- DOI: 10.1016/j.isci.2024.110953
Platelet factors ameliorate thoracic aortic aneurysm and dissection by inhibiting the FGF-FGFR cascade activation in aortic-endothelial cell
Abstract
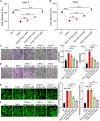
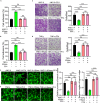
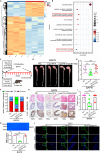
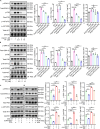
Thoracic aortic aneurysm and dissection (TAAD) is closely associated with vascular endothelial dysfunction. Platelet factor 4 (PF4) is crucial for maintaining vascular endothelial cell homeostasis. However, whether PF4 can influence the progression of TAAD remains unknown. In the present study, we constructed a liposome-encapsulated PF4 nanomedicine and verified its effect on BAPN-induced TAAD in vivo. We found that liposome PF4 nanoparticles (Lipo-PF4), more effectively than PF4 alone, inhibited the formation of TAAD. In vitro, PF4 improved endothelial cell function under pathological conditions by inhibiting migratory and angiogenic abilities of human aortic endothelial cells (HAECs). Mechanically, PF4 inhibited the development of TAAD and improved HAECs function by combining with heparin sulfate and blocking fibroblast growth factor-fibroblast growth factor receptor (FGF-FGFR) signaling. Taken together, we developed a nano-drug (Lipo-PF4) that effectively ameliorates the progression of TAAD by improving endothelial function. Lipo-PF4 is expected to be a therapeutic option for TAAD in the future.
Keywords: Biological sciences; Cardiovascular medicine; Drug delivery system; Therapeutics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Halushka M.K., Angelini A., Bartoloni G., Basso C., Batoroeva L., Bruneval P., Buja L.M., Butany J., d'Amati G., Fallon J.T., et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovasc. Pathol. 2016;25:247–257. doi: 10.1016/j.carpath.2016.03.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

