COVID-19 Is a Coronary Artery Disease Risk Equivalent and Exhibits a Genetic Interaction With ABO Blood Type
- PMID: 39381876
- PMCID: PMC11495539
- DOI: 10.1161/ATVBAHA.124.321001
COVID-19 Is a Coronary Artery Disease Risk Equivalent and Exhibits a Genetic Interaction With ABO Blood Type
Abstract
Background: COVID-19 is associated with acute risk of major adverse cardiac events (MACE), including myocardial infarction, stroke, and mortality (all-cause). However, the duration and underlying determinants of heightened risk of cardiovascular disease and MACE post-COVID-19 are not known.
Methods: Data from the UK Biobank was used to identify COVID-19 cases (n=10 005) who were positive for polymerase chain reaction (PCR+)-based tests for SARS-CoV-2 infection (n=8062) or received hospital-based International Classification of Diseases version-10 (ICD-10) codes for COVID-19 (n=1943) between February 1, 2020 and December 31, 2020. Population controls (n=217 730) and propensity score-matched controls (n=38 860) were also drawn from the UK Biobank during the same period. Proportional hazard models were used to evaluate COVID-19 for association with long-term (>1000 days) risk of MACE and as a coronary artery disease risk equivalent. Additional analyses examined whether COVID-19 interacted with genetic determinants to affect the risk of MACE and its components.
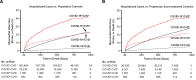
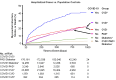
Results: The risk of MACE was elevated in COVID-19 cases at all levels of severity (HR, 2.09 [95% CI, 1.94-2.25]; P<0.0005) and to a greater extent in cases hospitalized for COVID-19 (HR, 3.85 [95% CI, 3.51-4.24]; P<0.0005). Hospitalization for COVID-19 represented a coronary artery disease risk equivalent since incident MACE risk among cases without history of cardiovascular disease was even higher than that observed in patients with cardiovascular disease without COVID-19 (HR, 1.21 [95% CI, 1.08-1.37]; P<0.005). A significant genetic interaction was observed between the ABO locus and hospitalization for COVID-19 (Pinteraction=0.01), with risk of thrombotic events being increased in subjects with non-O blood types (HR, 1.65 [95% CI, 1.29-2.09]; P=4.8×10-5) to a greater extent than subjects with blood type O (HR, 0.96 [95% CI, 0.66-1.39]; P=0.82).
Conclusions: Hospitalization for COVID-19 represents a coronary artery disease risk equivalent, with post-acute myocardial infarction and stroke risk particularly heightened in non-O blood types. These results may have important clinical implications and represent, to our knowledge, one of the first examples of a gene-pathogen exposure interaction for thrombotic events.
Keywords: COVID-19; SARS-CoV-2; genetics; major adverse cardiac events; myocardial infarction; stroke; thrombosis.
Conflict of interest statement
Dr Hazen reports being named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr Hazen also reports having received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Laboratory, a fully owned subsidiary of Quest Diagnostics, and Procter & Gamble. Dr Hazen is a paid consultant for Zehna Therapeutics and Proctor & Gamble and has received research funds from Zehna Therapeutics, Proctor & Gamble, Pfizer, and Roche Diagnostics. All other authors have no competing financial interests or personal relationships to declare related to the work reported in this article.
Figures

References
-
- WHO COVID-19 Dashboard. Geneva: World Health Organization; 2020. Accessed August 9, 2024. https://COVID19.who.int/
-
- Giacca M, Shah AM. The pathological maelstrom of COVID-19 and cardiovascular disease. Nat Cardiovasc Res. 2022;1:200–210. doi: 10.1038/s44161-022-00029-5 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

