Glycaemic outcomes in adults with type 2 diabetes over 34 weeks with the Omnipod® 5 Automated Insulin Delivery System
- PMID: 39382001
- PMCID: PMC11618232
- DOI: 10.1111/dom.15993
Glycaemic outcomes in adults with type 2 diabetes over 34 weeks with the Omnipod® 5 Automated Insulin Delivery System
Abstract
Aims: The aim was to evaluate the effect of extended use of the Omnipod® 5 Automated Insulin Delivery (AID) System in adults with type 2 diabetes and suboptimal glycaemic control.
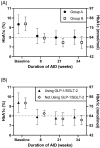
Materials and methods: Following an 8-week single-arm, multicentre, outpatient trial of AID in adults with type 2 diabetes and baseline HbA1c ≥ 8% (≥ 64 mmol/mol), participants were given the opportunity to continue use of the AID system in a 26-week (~6 month) extension phase. The primary safety endpoints were percentage of time with sensor glucose ≥ 250 mg/dL and < 54 mg/dL. Additional glycaemic measures, including percentage of time in range (TIR) (70-180 mg/dL) and HbA1c, were evaluated. The use of non-insulin anti-hyperglycaemic medications was permitted throughout the entire study.
Results: During the initial 8-week study, participants (N = 22) achieved a decrease in percentage of time ≥ 250 mg/dL from 27.4% ± 21.0% to 10.5% ± 8.8% (p < 0.0001), which further decreased to 9.7% ± 9.2% during the extension phase (p = 0.0002 vs. standard therapy). Percentage of time < 54 mg/dL remained low from standard therapy through extension (median [interquartile range] 0.00% [0.00%, 0.06%] vs. 0.02% [0.00%, 0.05%], p > 0.05). HbA1c decreased by 1.6% ± 1.2% (15.5 ± 13.1 mmol/mol, p < 0.0001) and TIR increased by 22.4% ± 19.2% (p < 0.0001) from standard therapy through extension with no significant change in body mass index and without an observed increase in total daily insulin requirements.
Conclusions: These longer-term findings of Omnipod 5 AID System use demonstrate the potential value of AID in helping people with type 2 diabetes reach glycaemic targets.
Keywords: GLP‐1; SGLT‐2 inhibitor; clinical trial; insulin pump therapy; type 2 diabetes.
© 2024 Insulet Corporation and The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Georgia M. Davis reports research support from Insulet and has served as a consultant for Medscape. Anne L. Peters served on an advisory board for Medscape and Vertex. Anne L. Peters has received research support from Insulet and Abbott Diabetes Care. She has stock options in Omada Health. Bruce W. Bode reports research support from Insulet during the conduct of the study, as well as research support from Abbott, Advance, Diasome, Dexcom, Janssen, Lilly, Medtronic, Novo Nordisk, Provention Bio, Sanofi, Sanvita, Senseonics, REMD Biotherapeutics, Xeris and vTv Therapeutics. Bruce W. Bode reports consultant and speaking fees from Boehringer Ingelheim, Insulet, Lilly, Mannkind, Medtronic, Novo Nordisk, Sanofi, Senseonics, Sanofi, Xeris and Zealand. Anders L. Carlson is an advisory board member, has received consulting fees and has spoken for Mannkind, Medtronic and Sanofi. Anders L. Carlson reports research support from Insulet, Dexcom, Medtronic, Abbott, Sanofi, Lilly, Novo Nordisk and UnitedHealth. Anders L. Carlson is an employee of the International Diabetes Center at Park Nicollet. Bonnie Dumais, Todd E. Vienneau, Lauren M. Huyett and Trang T. Ly are full‐time employees of and own stock in Insulet Corporation..
Figures
References
-
- Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta‐analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431‐437. - PubMed
-
- Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long‐term trends in antidiabetes drug usage in the U.S.: real‐world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69‐78. - PubMed
-
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26(1):107‐139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

