CHA2DS2-VASc score and prior oral anticoagulant use on endovascular treatment for acute ischemic stroke
- PMID: 39382062
- PMCID: PMC11651198
- DOI: 10.1002/acn3.52217
CHA2DS2-VASc score and prior oral anticoagulant use on endovascular treatment for acute ischemic stroke
Abstract
Objective: We evaluated the effect of CHA2DS2-VASc score and prior use of oral anticoagulants (OACs) on endovascular treatment (EVT) in patients with acute ischemic stroke and atrial fibrillation (AF).
Methods: Patients with AF who received EVT in 353 centers in Japan (2018-2020) were included. The outcomes were symptomatic intracerebral hemorrhage (sICH), in-hospital mortality, functional independence, and successful and complete reperfusion. The effects of CHA2DS2-VASc score, its components, and prior use of OACs were assessed via a multiple logistic regression model.
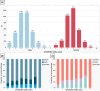
Results: Of the 6984 patients, 780 (11.2%) used warfarin and 1168 (16.7%) used direct oral anticoagulants (DOACs) before EVT. Based on the CHA2DS2-VASc score, 6046 (86.6%) presented a high risk (≥2 for males and ≥3 for females) while 938 (13.4%) had intermediate to low risks. Higher CHA2DS2-VASc scores were associated with increased sICH, in-hospital mortality, and decreased functional independence, regardless of prior OACs. For patients with a high-risk category, prior DOACs increased the odds of successful and complete reperfusion (adjusted odds ratio [95% confidence interval (CI)], 1.27 [1.00-1.61] and 1.30 [1.10-1.53]). For those with integrated intermediate to low risks, neither prior warfarin nor DOAC affected the outcomes. Regardless of total CHA2DS2-VASc scores, patients with congestive heart failure or left ventricular dysfunction, hypertension, age >75 years, or female benefited similarly from prior DOAC use.
Interpretation: Prior DOAC use for patients with high- and selected intermediate-risk CHA2DS2-VASc scores increased prevalence of successful and complete reperfusion. These findings may provide supplemental evidence to introduce preventive DOAC for patients with AF.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Dr. Imamura: speakers' bureau/honoraria from Medtronic Japan Co. (which own patent rights to EVT device), Daiichi Sankyo Co. (which own patent rights to DOAC edoxaban), Johnson & Johnson Co. (which own patent rights to EVT device), Stryker Japan Co. (which own patent rights to EVT device), Terumo Co. (which own patent rights to EVT device), and Asahi Intecc Co. (which own patent rights to EVT device). Dr. Ihara: lecturer's fees from Daiichi Sankyo Co. and Eisai (which own patent rights to warfarin), and Bristol‐Myers Squibb (which own patent rights to DOAC apixaban). Dr. Kamiyama: lecturer's fees from Daiichi Sankyo Co. Dr. Ohta reported lecturer's fees from Medtronic, Daiichi‐Sankyo, Johnson & Johnson, Terumo, Stryker Japan, Tokai Medical (which own patent rights to EVT device), Otsuka (which own patent rights to warfarin), Takeda (which own patent rights to warfarin), Eisai (which own patent rights to anticoagulant antagonist), Kaneka (which own patent rights to EVT device), Bristol‐Myers Squibb, AstraZeneca (which own patent rights to anticoagulant antagonist), Japan Lifeline (which own patent rights to EVT device), Nipro (which own patent rights to EVT device), and Century Medical (which own patent rights to EVT device), as well as consulting fees for Johnson & Johnson and Tokai Medical. Dr. Endo: lecturer's fees from Daiichi Sankyo. Dr Matsumaru: lecturer fees from Medtronic, Stryker, Terumo, Kaneka, and Daiichi Sakyo. Dr Sakai: a research grant from Japan Lifeline, Kaneka, Medtronic, Terumo, and TG Medical (which own patent rights to EVT device); lecturer's fees from Asahi‐Intec, Kaneka, Medtronic, Stryker, and Terumo; membership on the advisory boards for Johnson & Johnson, Medtronic, and Terumo. Dr. Kitazono: lecturer's fees and a research grant from Daiichi Sankyo. The remaining authors declare no conflicts of interest.
Figures


References
-
- Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857‐867. - PubMed
-
- Quinn GR, Severdija ON, Chang Y, Singer DE. Wide variation in reported rates of stroke across cohorts of patients with atrial fibrillation. Circulation. 2017;135:208‐219. - PubMed
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart Rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42:373‐498. - PubMed
-
- Anjum M, Ariansen I, Hjellvik V, et al. Stroke and bleeding risk in atrial fibrillation with CHA2DS2‐VASC risk score of one: the Norwegian AFNOR study. Eur Heart J. 2024;45:57‐66. - PubMed
MeSH terms
Substances
Grants and funding
- JP20ek0210129/Japan Agency for Medical Research and Development
- JP19ek0210088/Japan Agency for Medical Research and Development
- JP20ek0210147/Japan Agency for Medical Research and Development
- JP21ek0210147/Japan Agency for Medical Research and Development
- JP22ek0210147/Japan Agency for Medical Research and Development
- 20-4-10/Intramural Research Fund for Cardiovascular Diseases of National Cerebral and Cardiovascular Center
- 19AC1003/the Japanese Ministry of Health, Labour and Welfare
- 21FA1010/the Japanese Ministry of Health, Labour and Welfare
- 22FA1015/the Japanese Ministry of Health, Labour and Welfare
- 23FA1014/the Japanese Ministry of Health, Labour and Welfare
- 24FA1015/the Japanese Ministry of Health, Labour and Welfare
- 24FA1016/the Japanese Ministry of Health, Labour and Welfare
- H28-Shinkin-Ippan-011/the Japanese Ministry of Health, Labour and Welfare
- 22H03191/Japan Society for the Promotion of Science
- 18H02914/Japan Society for the Promotion of Science
- 23K24450/Japan Society for the Promotion of Science
- 25293314/Japan Society for the Promotion of Science
LinkOut - more resources
Full Text Sources
Medical

