Zelenirstat Inhibits N-Myristoyltransferases to Disrupt Src Family Kinase Signaling and Oxidative Phosphorylation, Killing Acute Myeloid Leukemia Cells
- PMID: 39382188
- PMCID: PMC11694064
- DOI: 10.1158/1535-7163.MCT-24-0307
Zelenirstat Inhibits N-Myristoyltransferases to Disrupt Src Family Kinase Signaling and Oxidative Phosphorylation, Killing Acute Myeloid Leukemia Cells
Abstract
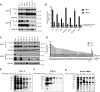
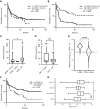
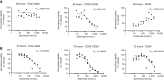
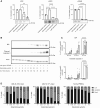
Acute myeloid leukemia (AML) is a hematologic malignancy with limited treatment options and a high likelihood of recurrence after chemotherapy. We studied N-myristoylation, the myristate modification of proteins linked to survival signaling and metabolism, as a potential therapeutic target for AML. N-myristoylation is catalyzed by two N-myristoyltransferases (NMT), NMT1 and NMT2, with varying expressions in AML cell lines and patient samples. We identified NMT2 expression as a marker for survival of patients with AML, and low NMT2 expression was associated with poor outcomes. We used the first-in-class pan-NMT inhibitor, zelenirstat, to investigate the role of N-myristoylation in AML. Zelenirstat effectively inhibits myristoylation in AML cell lines and patient samples, leading to degradation of Src family kinases, induction of endoplasmic reticulum stress, apoptosis, and cell death. Zelenirstat was well tolerated in vivo and reduced the leukemic burden in an ectopic AML cell line and in multiple orthotopic AML patient-derived xenograft models. The leukemia stem cell-enriched fractions of the hierarchical OCI-AML22 model were highly sensitive to myristoylation inhibition. Zelenirstat also impairs mitochondrial complex I and oxidative phosphorylation, which are critical for leukemia stem cell survival. These findings suggest that targeting N-myristoylation with zelenirstat represents a novel therapeutic approach for AML, with promise in patients with currently poor outcomes.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.M. Gamma reports grants from Alberta Cancer Foundation, Leukemia & Lymphoma Society of Canada, Award in Hematological Cancers’ Research in Memory of Dr. Rachel Mandel, Alberta Paving Ltd., Dr. Heleen & Rod McLeod, Eusera, and Pacylex Pharmaceuticals during the conduct of the study and personal fees from Pacylex Pharmaceuticals outside the submitted work, as well as a pending US patent application #63/573,885 assigned to Pacylex Pharmaceuticals, and is a current shareholder of Pacylex Pharmaceuticals. Q. Liu reports personal fees from Revolution Medicines outside the submitted work. E. Beauchamp reports personal fees from Pacylex Pharmaceuticals during the conduct of the study and personal fees from Pacylex Pharmaceuticals outside the submitted work, as well as a pending US patent application #63/573,885 assigned to Pacylex Pharmaceuticals, and patents #11,135,218 and #11,788,145 issued to Pacylex Pharmaceuticals Inc. M.C. Yap reports grants and personal fees from University of Alberta during the conduct of the study, as well as patents #11,135,218 and #11,788,145 issued to Pacylex Pharmaceuticals Inc. C. Ekstrom reports grants from Alberta Cancer Foundation and Leukemia & Lymphoma Society of Canada during the conduct of the study. R. Pain reports grants from Leukemia & Lymphoma Society of Canada, Alberta Cancer Foundation, Award in Hematological Cancers’ Research in Memory of Dr. Rachel Mandel, Alberta Paving Ltd., Dr. Heleen and Rod McLeod, Eusera, and Pacylex Pharmaceuticals Inc. during the conduct of the study, as well as a convertible note for investment into Pacylex. M.A. Kostiuk reports grants from Dr. Heleen and Rod McLeod through the Cancer Research Institute of Northern Alberta during the conduct of the study, as well as other support from Pacylex Pharmaceuticals Inc. outside the submitted work. J.R. Mackey reports other support from Pacylex Pharmaceuticals during the conduct of the study, as well as other support from illumiSonics outside the submitted work. J. Brandwein reports personal fees from AbbVie, Astellas, Bristol Myers and Squibb, Pfizer, Servier, Jazz, Taiho, and Amgen outside the submitted work. J.C.Y. Wang reports grants from University of Alberta during the conduct of the study, as well as other support from Pfizer and grants from Leukemia & Lymphoma Society of Canada outside the submitted work. L.G. Berthiaume reports personal fees and other support from Pacylex Pharmaceuticals Inc. (Pacylex) outside the submitted work, as well as pending US patent applications #63/573,885 and #63/093,970 assigned to Pacylex Pharmaceuticals Inc., and patents #11,135,218 and #11,788,145 issued to Pacylex Pharmaceuticals Inc. No disclosures were reported by the other authors.
Figures


References
-
- SEER*Explorer Application . [cited 2023 Dec 14]. Available from:https://seer.cancer.gov/statistics-network/explorer/application.html?sit....
-
- Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, et al. . Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov 2020;10:506–25. - PubMed
-
- Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, et al. . Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017;547:104–8. - PubMed
-
- Kamps MP, Buss JE, Sefton BM. Rous sarcoma virus transforming protein lacking myristic acid phosphorylates known polypeptide substrates without inducing transformation. Cell 1986;45:105–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

