Classics in Chemical Neuroscience: Tianeptine
- PMID: 39382192
- PMCID: PMC11587517
- DOI: 10.1021/acschemneuro.4c00519
Classics in Chemical Neuroscience: Tianeptine
Abstract
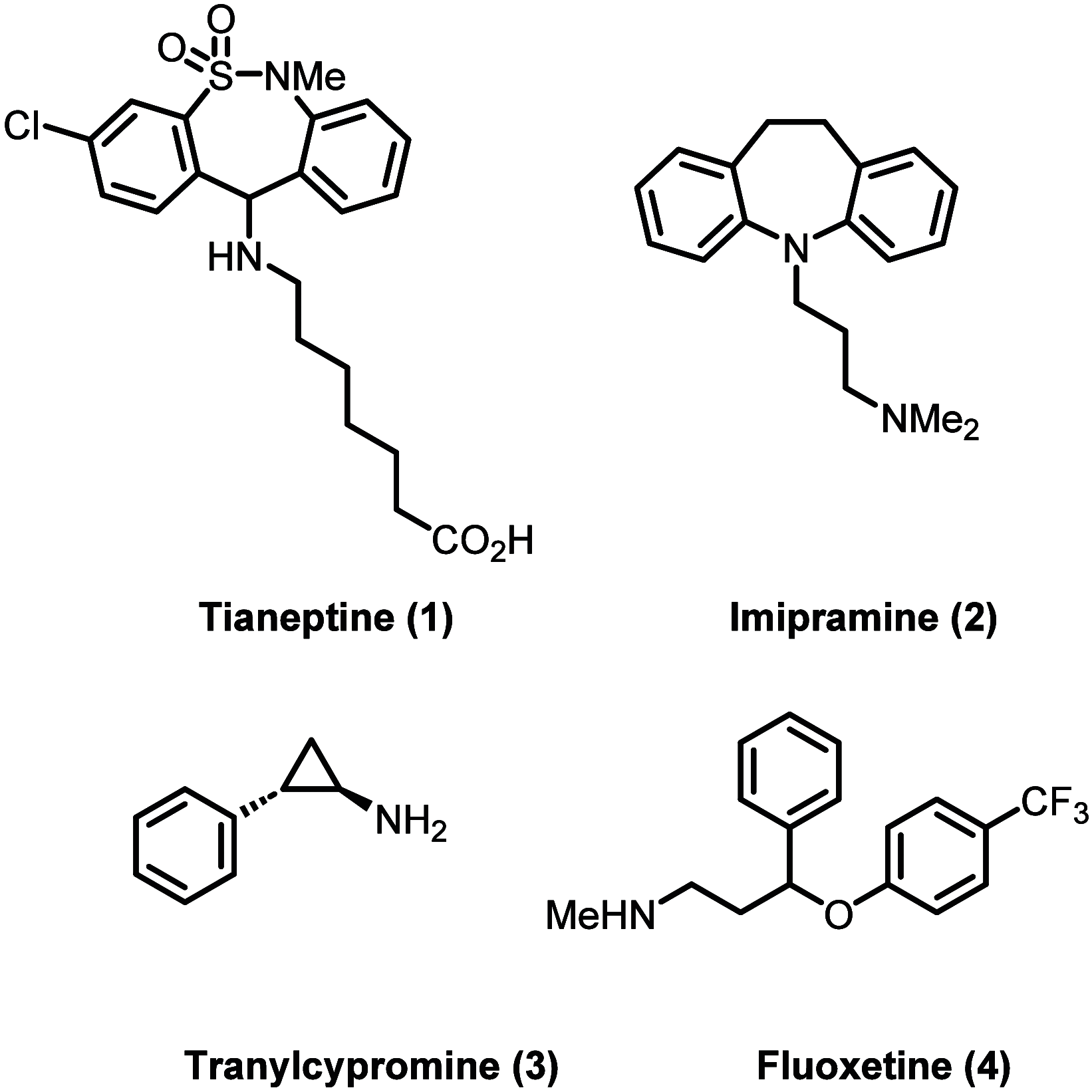
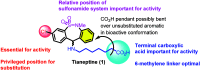
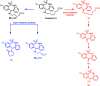
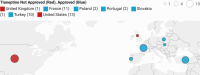
Tianeptine (1) is an unusual antidepressant in that its mechanism of action appears to be independent from any activity at serotonin receptors or monoamine transporters. In fact, tianeptine has been shown to be a moderately potent agonist for the mu opioid receptor (MOR) and to a lesser extent the delta opioid receptor (DOR). Additionally, tianeptine's efficacy may be related to its action on glutamate-mediated pathways of neuroplasticity. Regardless of which neurotransmitter system is primarily responsible for the observed efficacy, the MOR agonist activity is problematic with respect to abuse liability. Increasing numbers of case reports have demonstrated that tianeptine is indeed being used recreationally at doses far beyond what are considered therapeutically relevant or safe, and scheduling reclassifications or outright bans on tianeptine products are ongoing around the world. It is the aim of this review to discuss the medicinal chemistry and pharmacology of tianeptine and to summarize this intriguing discrepancy between tianeptine's historical use as a safe and effective antidepressant and its emerging potential for abuse.
Keywords: antidepressant; drug abuse; opioid; serotonin; tianeptine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- World Health Organization. Depressive disorder (depression); https://www.who.int/news-room/fact-sheets/detail/depression (accessed 2024–03–11).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

