Frequency of Screening and Spontaneous Breathing Trial Techniques: A Randomized Clinical Trial
- PMID: 39382222
- PMCID: PMC11581551
- DOI: 10.1001/jama.2024.20631
Frequency of Screening and Spontaneous Breathing Trial Techniques: A Randomized Clinical Trial
Abstract
Importance: The optimal screening frequency and spontaneous breathing trial (SBT) technique to liberate adults from ventilators are unknown.
Objective: To compare the effects of screening frequency (once-daily screening vs more frequent screening) and SBT technique (pressure-supported SBT with a pressure support level that was >0-≤8 cm H2O and a positive end-expiratory pressure [PEEP] level that was >0-≤5 cm H2O vs T-piece SBT) on the time to successful extubation.
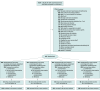
Design, setting, and participants: Randomized clinical trial with a 2 × 2 factorial design including critically ill adults who were receiving invasive mechanical ventilation for at least 24 hours, who were capable of initiating spontaneous breaths or triggering ventilators, and who were receiving a fractional concentration of inspired oxygen that was 70% or less and a PEEP level of 12 cm H2O or less. Recruitment was between January 2018 and February 2022 at 23 intensive care units in North America; last follow-up occurred October 18, 2022.
Interventions: Participants were enrolled early to enable protocolized screening (more frequent vs once daily) to identify the earliest that patients met criteria to undergo pressure-supported or T-piece SBT lasting 30 to 120 minutes.
Main outcome and measures: Time to successful extubation (time when unsupported, spontaneous breathing began and was sustained for ≥48 hours after extubation).
Results: Of 797 patients (198 in the once-daily screening and pressure-supported SBT group, 204 in once-daily screening and T-piece SBT, 195 in more frequent screening and pressure-supported SBT, and 200 in more frequent screening and T-piece SBT), the mean age was 62.4 (SD, 18.4) years and 472 (59.2%) were men. There were no statistically significant differences by screening frequency (hazard ratio [HR], 0.88 [95% CI, 0.76-1.03]; P = .12) or by SBT technique (HR, 1.06 [95% CI, 0.91-1.23]; P = .45). The median time to successful extubation was 2.0 days (95% CI, 1.7-2.7) for once-daily screening and pressure-supported SBT, 3.1 days (95% CI, 2.7-4.8) for once-daily screening and T-piece SBT, 3.9 days (95% CI, 2.9-4.7) for more frequent screening and pressure-supported SBT, and 2.9 days (95% CI, 2.0-3.1) for more frequent screening and T-piece SBT. An unexpected interaction between screening frequency and SBT technique required pairwise contrasts that revealed more frequent screening (vs once-daily screening) and pressure-supported SBT increased the time to successful extubation (HR, 0.70 [95% CI, 0.50-0.96]; P = .02). Once-daily screening and pressure-supported SBT (vs T-piece SBT) did not reduce the time to successful extubation (HR, 1.30 [95% CI, 0.98-1.70]; P = .08).
Conclusions and relevance: Among critically ill adults who received invasive mechanical ventilation for more than 24 hours, screening frequency (once-daily vs more frequent screening) and SBT technique (pressure-supported vs T-piece SBT) did not change the time to successful extubation. However, an unexpected and statistically significant interaction was identified; protocolized more frequent screening combined with pressure-supported SBTs increased the time to first successful extubation.
Trial registration: ClinicalTrials.gov Identifiers: NCT02399267 and NCT02969226.
Conflict of interest statement
Figures

Comment on
-
Ventilator Weaning Strategies-Managing Interaction Between Randomized Treatments.JAMA. 2024 Dec 3;332(21):1796-1797. doi: 10.1001/jama.2024.19853. JAMA. 2024. PMID: 39382243 No abstract available.
References
-
- MacIntyre NR, Cook DJ, Ely EW Jr, et al. ; American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine . Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6)(suppl):375S-395S. doi: 10.1378/chest.120.6_suppl.375S - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

