Restrictive vs Liberal Transfusion Strategy in Patients With Acute Brain Injury: The TRAIN Randomized Clinical Trial
- PMID: 39382241
- PMCID: PMC11581574
- DOI: 10.1001/jama.2024.20424
Restrictive vs Liberal Transfusion Strategy in Patients With Acute Brain Injury: The TRAIN Randomized Clinical Trial
Erratum in
-
Incorrect Author Name.JAMA. 2025 Mar 11;333(10):911. doi: 10.1001/jama.2025.1719. JAMA. 2025. PMID: 39946143 Free PMC article. No abstract available.
Abstract
Importance: Blood transfusions are commonly administered to patients with acute brain injury. The optimal hemoglobin transfusion threshold is uncertain in this patient population.
Objective: To assess the impact on neurological outcome of 2 different hemoglobin thresholds to guide red blood cell transfusions in patients with acute brain injury.
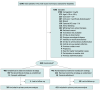
Design, setting, and participants: Multicenter, phase 3, parallel-group, investigator-initiated, pragmatic, open-label randomized clinical trial conducted in 72 intensive care units across 22 countries. Eligible patients had traumatic brain injury, aneurysmal subarachnoid hemorrhage, or intracerebral hemorrhage; hemoglobin values below 9 g/dL within the first 10 days after injury; and an expected intensive care unit stay of at least 72 hours. Enrollment occurred between September 1, 2017, and December 31, 2022. The last day of follow-up was June 30, 2023.
Interventions: Eight hundred fifty patients were randomly assigned to undergo a liberal (transfusion triggered by hemoglobin <9 g/dL; n = 408) or a restrictive (transfusion triggered by hemoglobin <7 g/dL; n = 442) transfusion strategy over a 28-day period.
Main outcomes and measures: The primary outcome was occurrence of an unfavorable neurological outcome, defined as a Glasgow Outcome Scale Extended score between 1 and 5, at 180 days following randomization. There were 14 prespecified serious adverse events, including occurrence of cerebral ischemia after randomization.
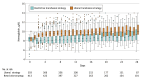
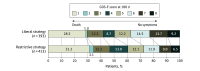
Results: Among 820 patients who completed the trial (mean age, 51 years; 376 [45.9%] women), 806 had available data on the primary outcome, 393 in the liberal strategy group and 413 in the restrictive strategy group. The liberal strategy group received a median of 2 (IQR, 1-3) units of blood, and the restrictive strategy group received a median of 0 (IQR, 0-1) units of blood, with an absolute mean difference of 1.0 unit (95% CI, 0.87-1.12 units). At 180 days after randomization, 246 patients (62.6%) in the liberal strategy group had an unfavorable neurological outcome compared with 300 patients (72.6%) in the restrictive strategy group (absolute difference, -10.0% [95% CI, -16.5% to -3.6%]; adjusted relative risk, 0.86 [95% CI, 0.79-0.94]; P = .002). The effect of the transfusion thresholds on neurological outcome at 180 days was consistent across prespecified subgroups. In the liberal strategy group, 35 (8.8%) of 397 patients had at least 1 cerebral ischemic event compared with 57 (13.5%) of 423 in the restrictive strategy group (relative risk, 0.65 [95% CI, 0.44-0.97]).
Conclusions and relevance: Patients with acute brain injury and anemia randomized to a liberal transfusion strategy were less likely to have an unfavorable neurological outcome than those randomized to a restrictive strategy.
Trial registration: ClinicalTrials.gov Identifier: NCT02968654.
Conflict of interest statement
Figures

Comment in
-
Shifting Balance of the Risk-Benefit of Restrictive Transfusion Strategies in Neurocritically Ill Patients-Is Less Still More?JAMA. 2024 Nov 19;332(19):1615-1617. doi: 10.1001/jama.2024.20416. JAMA. 2024. PMID: 39382236 No abstract available.
References
-
- Hébert PC, Wells G, Blajchman MA, et al. ; Transfusion Requirements in Critical Care Investigators; Canadian Critical Care Trials Group . A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi: 10.1056/NEJM199902113400601 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

