The alphaherpesvirus gE/gI glycoprotein complex and proteases jointly orchestrate invasion across the host's upper respiratory epithelial barrier
- PMID: 39382295
- PMCID: PMC11558996
- DOI: 10.1128/mbio.01873-24
The alphaherpesvirus gE/gI glycoprotein complex and proteases jointly orchestrate invasion across the host's upper respiratory epithelial barrier
Abstract
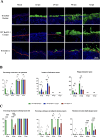
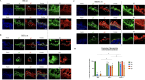
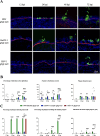
Alphaherpesviruses, including herpes simplex virus type 1 (HSV-1), pseudorabies virus (PRV), and bovine herpesvirus type 1 (BoHV-1), are significant pathogens affecting humans and animals. These viruses penetrate the upper respiratory tract mucosa, yet the mechanisms facilitating this invasion are not fully understood. This study investigates the role of the gE/gI glycoprotein complex and proteases in mucosal invasion by these viruses. Using species-specific respiratory mucosal explants, we observed that the removal of extracellular calcium disrupts epithelial junction integrity, enhancing viral infection across all viruses and suggesting a common mechanism of targeting a basolaterally located receptor. PRV exhibited significantly faster replication and deeper invasion compared to HSV-1 and BoHV-1. The gE glycoprotein was consistently polarized at the basement membrane across all viruses, indicating a critical role in the process of viral entry and subsequent spread through the epithelium. In this context, "infection" refers to the virus's attachment to its cell-surface receptor, entry into the cell, and completion of the viral life cycle, culminating in the production of progeny virions. Notably, in gE/gI null mutants of PRV and HSV-1, while the infection was not abortive and the viral life cycle was completed, the infection was delayed, and the invasion into the deeper layers of the epithelium and underlying mucosa was significantly reduced. In BoHV-1 mutants, this effect was even more pronounced, with infection restricted to the apical cells, failing to progress to the basal cells. In addition, PRV and HSV-1 invasion involved serine protease activity, unlike BoHV-1, which correlates with its slower invasion pace. Notably, the protease facilitating PRV invasion was identified as a urokinase plasminogen activator (uPA), while the specific protease for HSV-1 remains unidentified. These findings highlight the critical roles of the gE/gI complex and proteases in alphaherpesvirus pathogenesis, offering potential targets for therapeutic intervention.
Importance: Herpes simplex virus type 1 (HSV-1) infections are a worldwide issue. More than three billion people are infected with HSV-1 globally. Although most infections with HSV-1 occur subclinically, severe symptoms and complications are numerous and can be life-threatening. Complications include encephalitis and blindness. Recently, HSV-1 infections have been associated with the development of Alzheimer's Disease. To date, no effective vaccines against HSV-1 are on the market. Pseudorabies virus (PRV) and bovine herpesvirus type 1 (BoHV-1) are two alphaherpesviruses of major veterinary importance. Although efforts have been made to eradicate these viruses from livestock animals, clinical problems still occur, resulting in great economic losses for farmers. It is evident that new insights into the pathogenesis of alphaherpesviruses are needed, to develop effective treatments and novel preventive therapies.
Keywords: alphaherpesvirus; gE/gI complex; pathogenesis, mucosa invasion; proteases; upper respiratory tract; urokinase plasminogen activator.
Conflict of interest statement
G.A.S. is a co-founder of Thyreos, Inc., which is producing recombinant herpesvirus vaccines, and serves on the scientific advisory board of EG427. G.A.S. has stock ownership in both entities.
Figures




References
-
- Ajith Kumar AK, Mendez MD. 2023. Herpes simplex encephalitis. In StatPearls. StatPearls Publishing, Treasure Island (FL). - PubMed
-
- Bu X-L, Yao X-Q, Jiao S-S, Zeng F, Liu Y-H, Xiang Y, Liang C-R, Wang Q-H, Wang X, Cao H-Y, Yi X, Deng B, Liu C-H, Xu J, Zhang L-L, Gao C-Y, Xu Z-Q, Zhang M, Wang L, Tan X-L, Xu X, Zhou H-D, Wang Y-J. 2015. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol 22:1519–1525. doi: 10.1111/ene.12477 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

