GPR35 agonists inhibit TRPA1-mediated colonic nociception through suppression of substance P release
- PMID: 39382322
- PMCID: PMC11808708
- DOI: 10.1097/j.pain.0000000000003399
GPR35 agonists inhibit TRPA1-mediated colonic nociception through suppression of substance P release
Abstract
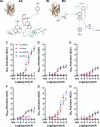
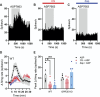
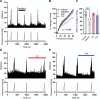
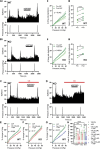
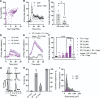
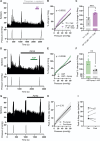
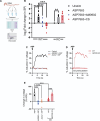
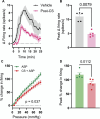
The development of nonopioid analgesics for the treatment of abdominal pain is a pressing clinical problem. To address this, we examined the expression of G i/o -coupled receptors, which typically inhibit nociceptor activation, in colonic sensory neurons. This led to the identification of the orphan receptor GPR35 as a visceral analgesic drug target because of its marked coexpression with transient receptor potential ankyrin 1 (TRPA1), a mediator of noxious mechanotransduction in the bowel. Building on in silico docking simulations, we confirmed that the mast cell stabiliser, cromolyn (CS), and phosphodiesterase inhibitor, zaprinast, are agonists at mouse GPR35, promoting the activation of different G i/o subunits. Pretreatment with either CS or zaprinast significantly attenuated TRPA1-mediated colonic nociceptor activation and prevented TRPA1-mediated mechanosensitisation. These effects were lost in tissue from GPR35 -/- mice and were shown to be mediated by inhibition of TRPA1-evoked substance P (SP) release. This observation highlights the pronociceptive effect of SP and its contribution to TRPA1-mediated colonic nociceptor activation and sensitisation. Consistent with this mechanism of action, we confirmed that TRPA1-mediated colonic contractions evoked by SP release were abolished by CS pretreatment in a GPR35-dependent manner. Our data demonstrate that GPR35 agonists prevent the activation and sensitisation of colonic nociceptors through the inhibition of TRPA1-mediated SP release. These findings highlight the potential of GPR35 agonists to deliver nonopioid analgesia for the treatment of abdominal pain.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
Dr Rie Suzuki and Dr Alastair Brown are employed by Sosei-Heptares. Dr David Bulmer receives research funding from Sosei-Heptares and GlaxoSmithKline.
Figures

References
-
- Balemans D, Aguilera-Lizarraga J, Florens MV, Jain P, Denadai-Souza A, Viola MF, Alpizar YA, Van Der Merwe S, Vanden Berghe P, Talavera K, Vanner S, Wouters MM, Boeckxstaens GE. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastro Liver Physiol 2019;316:G338–49. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004;41:849–57. - PubMed
-
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004;126:693–702. - PubMed
-
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007;132:26–37. - PubMed
MeSH terms
Substances
Grants and funding
- NF\R2\212001/Royal Society
- BB/M01194/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/W014831/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R006210/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/W002426/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

