Low-dose methadone added to another opioid for cancer pain: a multicentre prospective study
- PMID: 39382714
- PMCID: PMC11481640
- DOI: 10.1007/s00520-024-08835-2
Low-dose methadone added to another opioid for cancer pain: a multicentre prospective study
Abstract
Context: The use of methadone for cancer pain management is gaining wider acceptance. However, switching to methadone treatment can still pose challenges. Consequently, there is ongoing development of its use in low doses in combination with other opioids, despite a lack of clinical evidence regarding its efficacy and safety.
Objectives: This study aimed to evaluate the efficacy and tolerability of low-dose methadone in combination with another opioid in patients with moderate-to-severe cancer-related pain in a clinical setting.
Patients and methods: This was a prospective, open-label study conducted in 19 pain and/or palliative care centres treating patients with cancer-related pain. Pain intensity, patients' global impression of change, and adverse effects were assessed on day 7 and day 14. The main outcome measure was the proportion of responders.
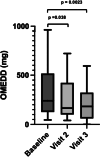
Results: The study included 92 patients. The daily dose of methadone was 3 [3-6] mg at baseline, 9 [4-10] mg on day 7 and 10 [6-15] mg on day 14. The NRS pain ratings significantly decreased from 7 [6-8] at baseline to 5 [3-6] on visit 2 (p < .0001) and 4 [3-6] on visit 3 (p < .0001). Similarly, the VRS pain ratings decreased from 3 [3-3] at baseline to 2 [2-3] on visit 2 (p = 0.026) and 2 [1-3] (p < 0.001) on visit 3. At Visits 1 and 2, half of the patients were considered Responders. Of those responders, 73.5% were High-Responders at Visit 1 and 58.7% were High-Responders at Visit 2. No adverse events related to the risk of QT prolongation, overdose, or drug interactions were reported.
Conclusion: For patients experiencing moderate to severe cancer-related pain despite initial opioid treatment, our study found that low-dose methadone, when used in combination with another opioid, was both safe and effective. This supports the use of methadone as an adjunct to opioid-based treatment for cancer pain.
Keywords: Cancer pain; Efficacy; Methadone co-analgesia; Titration; Tolerance.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

