Revising LH cut-off for the diagnosis of central precocious puberty via triptorelin stimulation assay
- PMID: 39382826
- PMCID: PMC11811446
- DOI: 10.1007/s12020-024-04055-0
Revising LH cut-off for the diagnosis of central precocious puberty via triptorelin stimulation assay
Abstract
Introduction: Precocious puberty (PP) in girls is defined by thelarche before age 8. The diagnostic gold standard is an increased LH level following gonadotropin-releasing hormone (GnRH) stimulation. Alternatively, GnRH analogues like triptorelin can be used, though their interpretation varies. Since 2000, we have used a triptorelin-induced LH cut-off of 15 IU/L, 4 h post-stimulus. However, many girls showed LH values below this threshold despite evident pubertal progression.
Purpose: To establish a new LH threshold post-triptorelin stimulation for earlier diagnosis of central precocious puberty (CPP) in girls showing pubertal progression and to evaluate additional parameters for diagnostic accuracy.
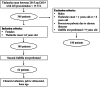
Methods: We enrolled 186 girls with thelarche onset between ages 1-8 and a GnRH analogue assay performed between 2015-2019 without signs of axis activation. Within this cohort, 62 patients repeated the triptorelin test due to rapid pubertal progression. The assay involved administering 100 mcg/m² of triptorelin and measuring LH, FSH, and estradiol levels before and four hours post-injection.
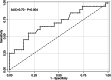
Results: Patients with axis activation at the second test had significantly higher post-stimulus LH levels at the first test compared to those below 15 IU/L. They also had higher basal LH levels, elevated LH/FSH ratio, and increased growth velocity. Statistical analysis identified a new post-stimulus LH threshold of 5 IU/L.
Conclusion: We propose a LH value of 5 IU/L after triptorelin administration as a new threshold for early CPP diagnosis. While the LH/FSH ratio and growth velocity are associated with axis activation, they did not significantly enhance diagnostic accuracy when combined with the LH value.
Keywords: Central precocious puberty; Cut-off; GnRH analogues; LH; Triptorelin Stimulation.
© 2024. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: The authors declare no competing interests. Ethical approval: The study was conducted in compliance with the terms of the Helsinki II Declaration and written informed consent for the enrolment and for the publication of individual clinical details was obtained from parents. The Institutional Ethics Committee of the province of Verona, Italy, took note of the retrospective conduct of the study.
Figures
References
-
- E.L. Zevin, E.A. Eugster, Central precocious puberty: a review of diagnosis, treatment, and outcomes. Lancet Child Adolesc Health. (2023) 10.1016/S2352-4642(23)00237-7 - PubMed
-
- M.M. Grumbach, D.M Styne, Puberty: ontogeny, neuroendocrinology, physiology. In: Williams Textbook of Endocrinology (Twelfth Edition), 1054-1201 (1998)
-
- P. Kaplowitz, C Bloch, Evaluation and referral of children with signs of early puberty. Pediatrics. (2016), 137 10.1542/peds.2015-3732 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous