Saurian-associated Leishmania tarentolae in dogs: Infectivity and immunogenicity evaluation in the canine model
- PMID: 39383180
- PMCID: PMC11463833
- DOI: 10.1371/journal.ppat.1012598
Saurian-associated Leishmania tarentolae in dogs: Infectivity and immunogenicity evaluation in the canine model
Abstract
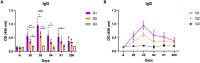
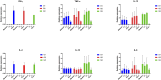
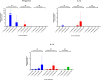
In canine leishmaniosis endemic areas, Leishmania infantum may occur in sympatry with the non-pathogenic Leishmania tarentolae, which is associated to reptiles. The potential infectivity of L. tarentolae for mammals raises questions about the interactions between the two Leishmania species, and the potential cross-immune protection in dogs. This study aimed to assess the outcome of experimental L. tarentolae infection in dogs, determining: i) the anti-L. tarentolae antibody production, ii) the duration of the immunity and cytokine expression, and iii) the possible pathogenic effect in the canine host. Twelve purpose-bred beagle dogs were randomly allocated to three groups (intravenous inoculation, G1; intradermal inoculation, G2; negative control, G3). G1 and G2 dogs were inoculated twice (day 0, day 28) with 108 promastigotes of L. tarentolae strain (RTAR/IT/21/RI-325) isolated from a Tarentola mauritanica gecko. The animals were followed until day 206. Blood, serum, conjunctival swabs and lymph node aspirate samples were collected monthly and bone marrow, liver and spleen biopsies on day 91. Hematological and biochemical parameters were assessed monthly, as well as serology (IFAT and ELISA) and molecular identification of L. tarentolae. Mononuclear cells (PBMC) were obtained to assess the cytokine expression through in vitro stimulation or (re-) infection. Data from this study demonstrated that DNA from L. tarentolae is detectable up to 3 months post-infection, with seroconversion after day 28. Moreover, the non-pathogenic nature of L. tarentolae was confirmed, with a neutral Th1/Th2 polarization, and a possible shift to Th1 phenotype after derived macrophages (re-) infection, as demonstrated by the expression of IFN-gamma. Therefore, L. tarentolae demonstrated a great potential as a surrogate pathogen and/or immune-prophylaxis/immune-therapy against Leishmania infections in dogs and humans.
Copyright: © 2024 Mendoza-Roldan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Martín-Sánchez J, Rodríguez-Granger J, Morillas-Márquez F, Merino-Espinosa G, Sampedro A, Aliaga L, et al.. Leishmaniasis due to Leishmania infantum: Integration of human, animal and environmental data through a One Health approach. Transbound Emerg Dis. 2020;67: 2423–2434. - PubMed
-
- Rêgo FD, Soares RP. Lutzomyia longipalpis: an update on this sand fly vector. An Acad Bras Cienc. 2021;93: e20200254. - PubMed

