Interleukin 11 therapy causes acute left ventricular dysfunction
- PMID: 39383190
- PMCID: PMC11687394
- DOI: 10.1093/cvr/cvae224
Interleukin 11 therapy causes acute left ventricular dysfunction
Abstract
Aims: Interleukin 11 (IL11) was initially thought important for platelet production, which led to recombinant IL11 being developed as a drug to treat thrombocytopenia. IL11 was later found to be redundant for haematopoiesis, and its use in patients is associated with unexplained and severe cardiac side effects. Here, we aim to identify, for the first time, direct cardiomyocyte toxicities associated with IL11, which was previously believed cardioprotective.
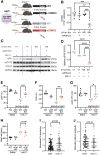
Methods and results: We injected recombinant mouse lL11 (rmIL11) into mice and studied its molecular effects in the heart using immunoblotting, qRT-PCR, bulk RNA-seq, single nuclei RNA-seq (snRNA-seq), and assay for transposase-accessible chromatin with sequencing (ATAC-seq). The physiological impact of IL11 was assessed by echocardiography in vivo and using cardiomyocyte contractility assays in vitro. To determine the activity of IL11 specifically in cardiomyocytes, we made two cardiomyocyte-specific Il11ra1 knockout (CMKO) mouse models using either AAV9-mediated and Tnnt2-restricted (vCMKO) or Myh6 (m6CMKO) Cre expression and an Il11ra1 floxed mouse strain. In pharmacologic studies, we studied the effects of JAK/STAT inhibition on rmIL11-induced cardiac toxicities. Injection of rmIL11 caused acute and dose-dependent impairment of left ventricular ejection fraction (saline: 62.4% ± 1.9; rmIL11: 32.6% ± 2.9, P < 0.001, n = 5). Following rmIL11 injection, myocardial STAT3 and JNK phosphorylation were increased and bulk RNA-seq revealed up-regulation of pro-inflammatory pathways (TNFα, NFκB, and JAK/STAT) and perturbed calcium handling. snRNA-seq showed rmIL11-induced expression of stress factors (Ankrd1, Ankrd23, Xirp2), activator protein-1 (AP-1) transcription factor genes, and Nppb in the cardiomyocyte compartment. Following rmIL11 injection, ATAC-seq identified the Ankrd1 and Nppb genes and loci enriched for stress-responsive, AP-1 transcription factor binding sites. Cardiomyocyte-specific effects were examined in vCMKO and m6CMKO mice, which were both protected from rmIL11-induced left ventricular impairment and molecular pathobiologies. In mechanistic studies, inhibition of JAK/STAT signalling with either ruxolitinib or tofacitinib prevented rmIL11-induced cardiac dysfunction.
Conclusions: Injection of IL11 directly activates IL11RA/JAK/STAT3 in cardiomyocytes to cause acute heart failure. Our data overturn the earlier assumption that IL11 is cardioprotective and explain the serious cardiac side effects associated with IL11 therapy.
Keywords: Cardiotoxicity; Fibrosis; Heart failure; Inflammation; Interleukin 11; JAK/STAT.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: S.A.C. is a co-inventor on a number of patent applications relating to the role of IL11 in human diseases that include the published patents: WO2017103108, WO2017103108 A2, WO 2018/109174 A2, and WO 2018/109170 A2. S.A.C. is also a co-founder and shareholder of Enleofen Bio PTE LTD and VVB PTE LTD.
Figures






Comment in
-
Inflammation and heart failure: are we facing a 'hedgehog's dilemma'?Cardiovasc Res. 2024 Dec 31;120(17):2155-2157. doi: 10.1093/cvr/cvae246. Cardiovasc Res. 2024. PMID: 39548817 No abstract available.
References
-
- Neben TY, Loebelenz J, Hayes L, McCarthy K, Stoudemire J, Schaub R, Goldman SJ. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood 1993;81:901–908. - PubMed
-
- Zhang J-J, Zhao R, Xia F, Li Y, Wang R-W, Guan X, Zhu J-G, Ma A-X. Cost-effectiveness analysis of rhTPO and rhIL-11 in the treatment of chemotherapy-induced thrombocytopenia in hematological tumors based on real-world data. Ann Palliat Med 2022;11:2709–2719. - PubMed
-
- Kaye JA. FDA licensure of NEUMEGA to prevent severe chemotherapy-induced thrombocytopenia. Stem Cells 1998;16:207–223. - PubMed
-
- Yu K-M, Lau JY-N, Fok M, Yeung Y-K, Fok S-P, Zhang S, Ye P, Zhang K, Li X, Li J, Xu Q, Wong W-T, Choo Q-L. Preclinical evaluation of the mono-PEGylated recombinant human interleukin-11 in cynomolgus monkeys. Toxicol Appl Pharmacol 2018;342:39–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- IAF-PP H23J2a0033/PREVENT-HF Industry Alignment Fund Pre-Positioning Programme
- Foundation Leducq
- NMRC/CG21APRC006/Collaborative Centre Grant scheme
- MC-A654-5QB30/MRC_/Medical Research Council/United Kingdom
- Singapore Ministry of Health's National Medical Research Council
- NMRC/STaR/0011/2012/National Medical Research Council Singapore STaR
- 203928/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- NMRC CG21APR1006/Centre Grant scheme
- Ministry of Health
- Duke-NUS-GCR/2015/0014/Goh Cardiovascular Research Award
- MOH-STaR21jun-0003/Singapore Translational Research Investigator Award
- National Institute of Health
- Duke-NUS Signature Research Programme
- Care Research Biomedical Research Centre Imperial College London
- WT_/Wellcome Trust/United Kingdom
- MC-A654-5QB10 /MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

