Loss of atrial natriuretic peptide signaling causes insulin resistance, mitochondrial dysfunction, and low endurance capacity
- PMID: 39383215
- PMCID: PMC11463261
- DOI: 10.1126/sciadv.adl4374
Loss of atrial natriuretic peptide signaling causes insulin resistance, mitochondrial dysfunction, and low endurance capacity
Abstract
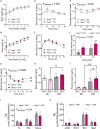
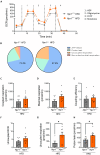
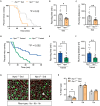
Type 2 diabetes (T2D) and obesity are strongly associated with low natriuretic peptide (NP) plasma levels and a down-regulation of NP guanylyl cyclase receptor-A (GCA) in skeletal muscle and adipose tissue. However, no study has so far provided evidence for a causal link between atrial NP (ANP)/GCA deficiency and T2D pathogenesis. Here, we show that both systemic and skeletal muscle ANP/GCA deficiencies in mice promote metabolic disturbances and prediabetes. Skeletal muscle insulin resistance is further associated with altered mitochondrial function and impaired endurance running capacity. ANP/GCA-deficient mice exhibit increased proton leak and reduced content of mitochondrial oxidative phosphorylation proteins. We further show that GCA is related to several metabolic traits in T2D and positively correlates with markers of oxidative capacity in human skeletal muscle. Together, these results indicate that ANP/GCA signaling controls muscle mitochondrial integrity and oxidative capacity in vivo and plays a causal role in the development of prediabetes.
Figures





References
-
- Roden M., Shulman G. I., The integrative biology of type 2 diabetes. Nature 576, 51–60 (2019). - PubMed
-
- Walford G. A., Ma Y., Christophi C. A., Goldberg R. B., Jarolim P., Horton E., Mather K. J., Barrett-Connor E., Davis J., Florez J. C., Wang T. J., Circulating natriuretic peptide concentrations reflect changes in insulin sensitivity over time in the Diabetes Prevention Program. Diabetologia 57, 935–939 (2014). - PMC - PubMed
-
- Wang T. J., Larson M. G., Levy D., Benjamin E. J., Leip E. P., Wilson P. W., Vasan R. S., Impact of obesity on plasma natriuretic peptide levels. Circulation 109, 594–600 (2004). - PubMed
-
- Kuhn M., Molecular physiology of membrane guanylyl cyclase receptors. Physiol. Rev. 96, 751–804 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

