Bidirectional regulation of motor circuits using magnetogenetic gene therapy
- PMID: 39383230
- PMCID: PMC11463271
- DOI: 10.1126/sciadv.adp9150
Bidirectional regulation of motor circuits using magnetogenetic gene therapy
Abstract
Here, we report a magnetogenetic system, based on a single anti-ferritin nanobody-TRPV1 receptor fusion protein, which regulated neuronal activity when exposed to magnetic fields. Adeno-associated virus (AAV)-mediated delivery of a floxed nanobody-TRPV1 into the striatum of adenosine-2a receptor-Cre drivers resulted in motor freezing when placed in a magnetic resonance imaging machine or adjacent to a transcranial magnetic stimulation device. Functional imaging and fiber photometry confirmed activation in response to magnetic fields. Expression of the same construct in the striatum of wild-type mice along with a second injection of an AAVretro expressing Cre into the globus pallidus led to similar circuit specificity and motor responses. Last, a mutation was generated to gate chloride and inhibit neuronal activity. Expression of this variant in the subthalamic nucleus in PitX2-Cre parkinsonian mice resulted in reduced c-fos expression and motor rotational behavior. These data demonstrate that magnetogenetic constructs can bidirectionally regulate activity of specific neuronal circuits noninvasively in vivo using clinically available devices.
Figures


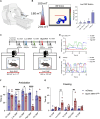

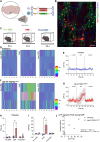
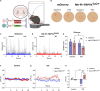

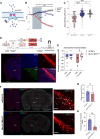
Update of
-
Bidirectional Regulation of Motor Circuits Using Magnetogenetic Gene Therapy Short: Magnetogenetic Regulation of Motor Circuits.bioRxiv [Preprint]. 2024 Apr 29:2023.07.13.548699. doi: 10.1101/2023.07.13.548699. bioRxiv. 2024. Update in: Sci Adv. 2024 Oct 11;10(41):eadp9150. doi: 10.1126/sciadv.adp9150. PMID: 37503198 Free PMC article. Updated. Preprint.
References
-
- Krauss J. K., Lipsman N., Aziz T., Boutet A., Brown P., Chang J. W., Davidson B., Grill W. M., Hariz M. I., Horn A., Schulder M., Mammis A., Tass P. A., Volkmann J., Lozano A. M., Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 17, 75–87 (2021). - PMC - PubMed
-
- Benabid A. L., Chabardes S., Mitrofanis J., Pollak P., Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 8, 67–81 (2009). - PubMed
-
- Boyden E. S., Zhang F., Bamberg E., Nagel G., Deisseroth K., Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

