LINE1 modulate human T cell function by regulating protein synthesis during the life span
- PMID: 39383231
- PMCID: PMC11463280
- DOI: 10.1126/sciadv.ado2134
LINE1 modulate human T cell function by regulating protein synthesis during the life span
Abstract
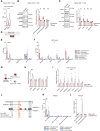
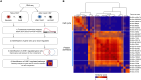
The molecular mechanisms responsible for the heightened reactivity of quiescent T cells in human early life remain largely elusive. Our previous research identified that quiescent adult naïve CD4+ T cells express LINE1 (long interspersed nuclear elements 1) spliced in previously unknown isoforms, and their down-regulation marks the transition to activation. Here, we unveil that neonatal naïve T cell quiescence is characterized by enhanced energy production and protein synthesis. This phenotype is associated with the absence of LINE1 expression attributed to tonic T cell receptor/mTOR complex 1 (mTORC1) signaling and (polypyrimidine tract-binding protein 1 (PTBP1)-mediated LINE1 splicing suppression. The absence of LINE1 expression primes these cells for rapid execution of the activation program by directly regulating protein synthesis. LINE1 expression progressively increases in childhood and adults, peaking in elderly individuals, and, by decreasing protein synthesis, contributes to immune senescence in aging. Our study proposes LINE1 as a critical player of human T cell function across the human life span.
Figures




References
-
- Lucivero G., D’Addario V., Tannoia N., Dell’Osso A., Gambatesa V., Lopalco P. L., Cagnazzo G., Ontogeny of human lymphocytes: Two-color fluorescence analysis of circulating lymphocyte subsets in fetuses in the second trimester of pregnancy. Fetal Diagn. Ther. 6, 101–106 (2009). - PubMed
-
- D'Arena G., Musto P., Cascavilla N., Di Giorgio G., Fusilli S., Zendoli F., Carotenuto M., Flow cytometric characterization of human umbilical cord blood lymphocytes: Immunophenotypic features. Haematologica 83, 197–203 (1998). - PubMed
-
- Fink P. J., The biology of recent thymic emigrants. Annu. Rev. Immunol. 31, 31–50 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

