SLC26A4-AP-2 mu2 interaction regulates SLC26A4 plasma membrane abundance in the endolymphatic sac
- PMID: 39383236
- PMCID: PMC11638888
- DOI: 10.1126/sciadv.adm8663
SLC26A4-AP-2 mu2 interaction regulates SLC26A4 plasma membrane abundance in the endolymphatic sac
Abstract
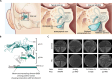
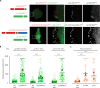
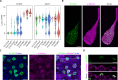
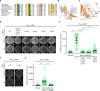
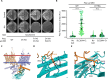
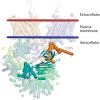
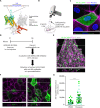
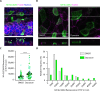
Decreased presence or activity of human SLC26A4 at the plasma membrane is a common cause of hearing loss. SLC26A4 (Pendrin) is necessary for normal reabsorption of endolymph, the fluid bathing the inner ear. We identified the μ2 subunit of adaptor protein 2 (AP-2) complex required for clathrin-mediated endocytosis as a protein-partner of SLC26A4 involved in regulating its plasma membrane abundance. We showed that, in the endolymphatic sac, where fluid reabsorption occurs, SLC26A4 is localized along the apical microvilli of mitochondria-rich cells, in contact with the endolymph, and associated with clathrin-coated pits where μ2 and AP-2 are present. Based on SLC26A4 structure, the elements involved in SLC26A4-μ2 interaction were identified and validated experimentally, allowing modeling of this interaction at the atomic level. Pharmacological inhibition of clathrin-mediated endocytosis led to an increased plasma membrane abundance of hemagglutinin-tagged SLC26A4 virally or endogenously expressed in mitochondria-rich cells. These results indicate that the SLC26A4-μ2 interaction regulates SLC26A4 abundance at the apical surface of mitochondria-rich cells.
Figures

References
-
- Dahlmann A., von During M., The endolymphatic duct and sac of the rat: A histological, ultrastructural, and immunocytochemical investigation. Cell Tissue Res. 282, 277–289 (1995). - PubMed
-
- Everett L. A., Glaser B., Beck J. C., Idol J. R., Buchs A., Heyman M., Adawi F., Hazani E., Nassir E., Baxevanis A. D., Sheffield V. C., Green E. D., Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat. Genet. 17, 411–422 (1997). - PubMed
-
- Luxon L. M., Cohen M., Coffey R. A., Phelps P. D., Britton K. E., Jan H., Trembath R. C., Reardon W., Neuro-otological findings in Pendred syndrome. Int. J. Audiol. 42, 82–88 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

