Collagenase Injection versus Limited Fasciectomy for Dupuytren's Contracture
- PMID: 39383454
- PMCID: PMC7616701
- DOI: 10.1056/NEJMoa2312631
Collagenase Injection versus Limited Fasciectomy for Dupuytren's Contracture
Abstract
Background: Treatments for Dupuytren's contracture include limited fasciectomy and collagenase injection. Comparisons of the effectiveness of these treatments have been limited.
Methods: We performed an unblinded, multicenter, pragmatic, two-group, randomized, controlled noninferiority trial comparing collagenase injection with limited fasciectomy in persons with moderate Dupuytren's contracture. The primary outcome was the score on the Patient Evaluation Measure-Hand Health Profile (PEM), a questionnaire for assessing hand health as reported by the patient, at 1 year after treatment. Scores on the PEM range from 0 to 100, with higher scores indicating worse outcomes. The prespecified noninferiority margin was 6 points.
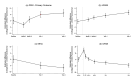
Results: A total of 672 persons (336 per group) were assigned to receive collagenase injection or to undergo limited fasciectomy. The primary analysis included 599 persons: 314 in the collagenase group and 285 in the limited-fasciectomy group. The mean score on the PEM at 1 year was 17.8 among the 284 patients with available data in the collagenase group and 11.9 among the 250 patients with available data in the limited-fasciectomy group (estimated difference, 5.9 points; 95% confidence interval [CI], 3.1 to 8.8; one-sided P = 0.49 for noninferiority). Among the patients with available data (229 patients in the collagenase group and 197 patients in the limited-fasciectomy group), the estimated difference in the mean score on the PEM at 2 years was 7.2 points (95% CI, 4.2 to 10.9). Moderate or severe complications of treatment occurred in 1.8% of the patients in the collagenase group and in 5.1% of those in the limited-fasciectomy group; recurrent contracture resulted in reintervention in 14.6% and 3.4%, respectively.
Conclusions: Collagenase injection was not noninferior to limited fasciectomy with respect to the score on the PEM at 1 year after treatment. (Funded by the National Institute for Health and Care Research Health Technology Assessment Programme; DISC ISRCTN Registry number ISRCTN18254597.).
Copyright © 2024 Massachusetts Medical Society.
Figures

Comment in
-
Treatments for Dupuytren's Contracture.N Engl J Med. 2025 Jan 23;392(4):10.1056/NEJMc2415105#sa1. doi: 10.1056/NEJMc2415105. N Engl J Med. 2025. PMID: 39842019 No abstract available.
-
Treatments for Dupuytren's Contracture.N Engl J Med. 2025 Jan 23;392(4):10.1056/NEJMc2415105#sa2. doi: 10.1056/NEJMc2415105. N Engl J Med. 2025. PMID: 39842020 No abstract available.
-
Treatments for Dupuytren's Contracture. Reply.N Engl J Med. 2025 Jan 23;392(4):10.1056/NEJMc2415105#sa3. doi: 10.1056/NEJMc2415105. N Engl J Med. 2025. PMID: 39842021 No abstract available.
References
-
- Huang C, Ogawa R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res. 2012;53:187–196. - PubMed
-
- NHS. Dupuytren’s contracture. 2021. [Accessed 27/04/2023 2023]. https://www.nhs.uk/conditions/dupuytrens-contracture/
-
- Gudmundsson KG, Arngrímsson R, Sigfússon N, et al. Epidemiology of Dupuytren’s disease: Clinical, serological, and social assessment. The Reykjavik Study. Journal of Clinical Epidemiology. 2000;53:291–296. - PubMed
-
- Dahlin L, Bainbridge C, Leclercq C, et al. Dupuytren’s disease presentation, referral pathways and resource utilisation in Europe: regional analysis of a surgeon survey and patient chart review. International journal of clinical practice. 2013;67:261–270. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
