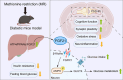
Methionine restriction alleviates diabetes-associated cognitive impairment via activation of FGF21
- PMID: 39383602
- PMCID: PMC11492615
- DOI: 10.1016/j.redox.2024.103390
Methionine restriction alleviates diabetes-associated cognitive impairment via activation of FGF21
Abstract
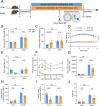
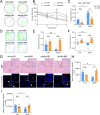
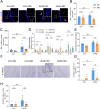
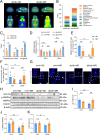
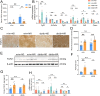
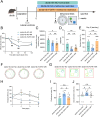
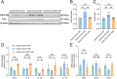
Glucose metabolism disturbances may result in diabetes-associated cognitive decline (DACI). Methionine restriction (MR) diet has emerged as a potential dietary strategy for managing glucose homeostasis. However, the effects and underlying mechanisms of MR on DACI have not been fully elucidated. Here, we found that a 13-week MR (0.17 % methionine, w/w) intervention starting at 8 weeks of age improved peripheral insulin sensitivity in male db/db mice, a model for type 2 diabetes. Notably, MR significantly improved working as well as long-term memory in db/db mice, accompanied by increased PSD-95 level and reduced neuroinflammatory factors, malondialdehyde (MDA), and 8-hydroxy-2'-deoxyguanosine (8-OHdG). We speculate that this effect may be mediated by MR activating hepatic fibroblast growth factor 21 (FGF21) and the brain FGFR1/AMPK/GLUT4 signaling pathway to enhance brain glucose metabolism. To further delineate the mechanism, we used intracerebroventricular injection of adeno-associated virus to specifically knock down FGFR1 in the brain to verify the role of FGFR1 in MR-mediated DACI. It was found that the positive effects of MR on DACI were offset, reflected in decreased cognitive function, impaired synaptic plasticity, upregulated neuroinflammation, and balanced enzymes regulating reactive oxygen species (Sod1, Sod2, Nox4). Of note, the FGFR1/AMPK/GLUT4 signaling pathway and brain glucose metabolism were inhibited. In summary, our study demonstrated that MR increased peripheral insulin sensitivity, activated brain FGFR1/AMPK/GLUT4 signaling through FGF21, maintained normal glucose metabolism and redox balance in the brain, and thereby alleviated DACI. These results provide new insights into the effects of MR diet on cognitive dysfunction caused by impaired brain energy metabolism.
Keywords: AMP-Activated protein kinase; Diabetes-associated cognitive impairment; Fibroblast growth factor 21; Glucose metabolism; Glucose transporter proteins; Methionine restriction.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

