Next-gen spinal cord injury clinical trials: lessons learned and opportunities for future success
- PMID: 39383609
- PMCID: PMC11490878
- DOI: 10.1016/j.ebiom.2024.105381
Next-gen spinal cord injury clinical trials: lessons learned and opportunities for future success
Abstract
Despite promising basic science discoveries and a surge in clinical trials, the quest for effective treatments that restore neurological function after spinal cord injury lags on. While "failed" in a conventional sense, emerging solutions to longstanding challenges represent promising steps towards a future with effective interventions. In this personal view, we highlight clinical trials implementing new solutions and their impact on the field. Our perspective is that, ultimately, the integration of shared knowledge, adaptive designs, and a deeper understanding of the intricacies of spinal cord injury holds promise of unlocking of major breakthroughs, leading to improved outcomes for people with spinal cord injury.
Keywords: Adaptive design; Neurorestorative trials; Spinal cord injury clinical trials.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests P.S.S. is a member of SCITrialsFinder curation group with no financial conflict of interest to declare. J.L.K.K. previously consulted for Axonis. The authors have no other conflicts of interest to declare.
Figures
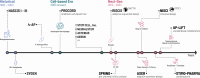
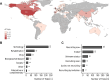

References
-
- Ahuja C.S., Wilson J.R., Nori S., et al. Traumatic spinal cord injury. Nat Rev Dis Prim. 2017;3:17018. - PubMed
-
- Dijkers M.P.J.M. Quality of life of individuals with spinal cord injury: a review of conceptualization, measurement, and research findings. J Rehabil Res Dev. 2005;42:87–110. - PubMed
-
- Boakye M., Leigh B.C., Skelly A.C. Quality of life in persons with spinal cord injury: comparisons with other populations. J Neurosurg Spine. 2012;17:29–37. - PubMed
-
- Dijkers M. Quality of life after spinal cord injury: a meta analysis of the effects of disablement components. Spinal Cord. 1997;35:829–840. - PubMed
-
- Krueger H., Noonan V.K., Trenaman L.M., Joshi P., Rivers C.S. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33:113–122. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

