Induction, amplification, and propagation of diabetic retinopathy-associated inflammatory cytokines between human retinal microvascular endothelial and Müller cells and in the mouse retina
- PMID: 39384004
- PMCID: PMC12188995
- DOI: 10.1016/j.cellsig.2024.111454
Induction, amplification, and propagation of diabetic retinopathy-associated inflammatory cytokines between human retinal microvascular endothelial and Müller cells and in the mouse retina
Abstract
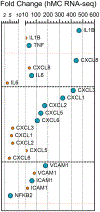
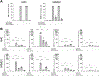
Ocular levels of IL-1β, TNFα, IL-8, and IL-6 correlate with progression of diabetic retinopathy (DR). Müller cells (MC), which are crucial to maintaining retinal homeostasis, are targets and sources of these cytokines. We explored the relative capacities of these four DR-associated cytokines to amplify inflammatory signal expression both in and between human MC (hMC) and retinal microvascular endothelial cells (hRMEC) and in the mouse retina. Of the four cytokines, IL-1β was the most potent stimulus of transcriptomic alterations in hMC and hRMEC in vitro, as well as in the mouse retina after intravitreal injection in vivo. Stimulation with IL-1β significantly induced expression of all four transcripts in hMC and hRMEC. TNFα significantly induced expression of some, but not all, of the four transcripts in each cell, while neither IL-8 nor IL-6 showed significant induction in either cell. Similarly, conditioned media (CM) derived from hMC or hRMEC treated with IL-1β, but not TNFα, upregulated inflammatory cytokine transcripts in the reciprocal cell type. hRMEC responses to hMC-derived CM were dependent on IL-1R activation. In addition, we observed a correlation between cytokine expression changes following direct and CM stimulation and NFκB-p65 nuclear translocation in both hMC and hRMEC. Finally, in mice, intravitreal injections of IL-1β, but not TNFα, induced retinal expression of Il1b and CXCL8 homologues Cxcl1, Cxcl2, Cxcl3, and Cxcl5, encoding pro-angiogenic chemokines. Our results suggest that expression of IL-1β, TNFα, IL-8, and IL-6 may be initiated, propagated, and sustained by autocrine and paracrine signals in hRMEC and hMC through a process involving IL-1β and NFκB. Targeting these signals may help thwart inflammatory amplification, preventing progression to vision-threatening stages and preserving sight.
Keywords: Chemokines; Conditioned media; Diabetic retinopathy; IL-1β; Inflammation; NFκB.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest There are no financial or non-financial competing interests declared by any author.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

