Dendritic cells pulsed with multifunctional Wilms' tumor 1 (WT1) peptides combined with multiagent chemotherapy modulate the tumor microenvironment and enable conversion surgery in pancreatic cancer
- PMID: 39384197
- PMCID: PMC11474828
- DOI: 10.1136/jitc-2024-009765
Dendritic cells pulsed with multifunctional Wilms' tumor 1 (WT1) peptides combined with multiagent chemotherapy modulate the tumor microenvironment and enable conversion surgery in pancreatic cancer
Abstract
Background: We aimed to develop a chemoimmunotherapy regimen consisting of a novel Wilms' tumor 1 (WT1) peptide-pulsed dendritic cell (WT1-DC) vaccine and multiagent chemotherapy and to investigate the safety, clinical outcomes, and WT1-specific immune responses of patients with unresectable advanced pancreatic ductal adenocarcinoma (UR-PDAC) who received this treatment.
Methods: Patients with UR-PDAC with stage III disease (locally advanced (LA-PDAC; n=6)), stage IV disease (metastatic (M-PDAC; n=3)), or recurrent disease after surgery (n=1) were enrolled in this phase I study. The patients received one cycle of nab-paclitaxel plus gemcitabine alone followed by 15 doses of the WT1-DC vaccine independent of chemotherapy. The novel WT1 peptide cocktail was composed of a multifunctional helper peptide specific for major histocompatibility complex class II, human leukocyte antigen (HLA)-A*02:01, or HLA-A*02:06 and a killer peptide specific for HLA-A*24:02.
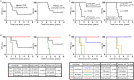
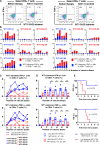
Results: The chemoimmunotherapy regimen was well tolerated. In the nine patients for whom a prognostic analysis was feasible, the clinical outcomes of long-term WT1 peptide-specific delayed-type hypersensitivity (WT1-DTH)-positive patients (n=4) were significantly superior to those of short-term WT1-DTH-positive patients (n=5). During chemoimmunotherapy, eight patients were deemed eligible for conversion surgery and underwent R0 resection (four patients with LA-PDAC, one patient with M-PDAC, and one recurrence) or R1 resection (one patient with M-PDAC), and one patient with LA-PDAC was determined to be unresectable. Long-term WT1-DTH positivity was observed in three of the four patients with R0-resected LA-PDAC. These three patients exhibited notable infiltration of T cells and programmed cell death protein-1+ cells within the pancreatic tumor microenvironment (TME). All patients with long-term WT1-DTH positivity were alive for at least 4.5 years after starting therapy. In patients with long-term WT1-DTH positivity, the percentage of WT1-specific circulating CD4+ or CD8+ T cells that produced IFN-γ or TNF-α was significantly greater than that in patients with short-term WT1-DTH positivity after two vaccinations. Moreover, after 12 vaccinations, the percentages of both circulating regulatory T cells and myeloid-derived suppressor cells were significantly lower in patients with long-term WT1-DTH-positive PDAC than in short-term WT1-DTH-positive patients.
Conclusions: Potent activation of WT1-specific immune responses through a combination chemoimmunotherapy regimen including the WT1-DC vaccine in patients with UR-PDAC may modulate the TME and enable conversion surgery, resulting in clinical benefits (Online supplemental file 1).
Trial registration number: jRCTc030190195.
Keywords: Dendritic; Immunotherapy; Tumor infiltrating lymphocyte - TIL; Tumor microenvironment - TME; Vaccine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Sugiyama H. WT1 cancer vaccine for the treatment and prevention. MRAJ. 2022;10:1–45. doi: 10.18103/mra.v10i4.2762. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
