Control of myopia using diffusion optics spectacle lenses: 4-year results of a multicentre randomised controlled, efficacy and safety study (CYPRESS)
- PMID: 39384223
- PMCID: PMC11733789
- DOI: 10.1136/bmjophth-2024-001790
Control of myopia using diffusion optics spectacle lenses: 4-year results of a multicentre randomised controlled, efficacy and safety study (CYPRESS)
Abstract
Aims: To evaluate the myopia control efficacy of Diffusion Optics Technology (DOT) spectacle lenses in children over a 4-year treatment period.
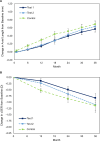
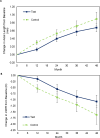
Methods: CYPRESS Part 1 (NCT03623074) was a 3-year multicentre, randomised, controlled, double-masked trial comparing two investigational spectacle lens DOT designs (Test 1, Test 2) and standard single vision Control lenses in 256 North American children aged 6-10 years. Children completing Part 1 (n=200) were invited to enrol in CYPRESS Part 2 (NCT04947735) for an additional 1-year period. In Part 2, Test 1 (n=35) and Control groups (n=42) continued with their original lens assignment and the Test 2 group (n=21) were crossed over to Test 1 (DOT 0.2) lenses. The co-primary endpoints were change from baseline in axial length (AL) and cycloplegic spherical equivalent refraction (cSER).
Results: Test 1 spectacle lenses demonstrated superiority to the Control in both co-primary endpoints: with a difference between means (Test 1-Control) of -0.13 mm for AL (p=0.018) and 0.33 D for cSER (p=0.008) in Part 1 and -0.05 mm for AL (p=0.038) and 0.13 D for cSER (p=0.043) in Part 2. Comparing treatment effects in Part 1 and 2 suggests that COVID-19 public health restrictions negatively impacted treatment efficacy in study years 2 and 3.
Conclusion: DOT 0.2 spectacle lenses are safe and effective at reducing myopia progression, with additional benefit evident in year 4 of wear. These results support the hypothesis that a mild reduction in retinal contrast can slow myopia progression in young children. The unprecedented disruption in participant schooling and lifestyle during the COVID-19 pandemic may have depressed treatment efficacy in Part 1.
Keywords: Child health (paediatrics); Clinical Trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DL, JSH, MMP and VT are SightGlass Vision employees. VT has a patent application for methods and devices for reducing myopia in children. JW reports research grants from SightGlass Vision, Alcon, CooperVision and Johnson and Johnson Vision and consultancy fees from SightGlass Vision and meeting and/or travel support from Alcon and CopperVision. XZ reports grants from SightGlass Vision and Reality Labs Research at Meta Platforms Technologies, LLC and consultancy fees/honoraria from SightGlass Vision and CooperVision. GY, RC and CH report contracts with SightGlass Vision, Johnson & Johnson Vision, CooperVision and Essilor. JN and MN report royalties/licenses, consulting fees and meeting and/or travel support from SightGlass Vision. JN and MN are cofounders and have stock ownership in SightGlass Vision, and are listed as Inventors on patents issued for DOT lens, owned by the University of Washington. TWC reports consulting fees, meeting and/or travel support, patents and stock ownership in his role as employee, officer and board member for SightGlass Vision. DJ reports consulting/honoraria from CooperVision, Hoya, SightGlass Vision, Alcon and Essilor. JSW reports consulting fees from CooperVision, DopaVision, Essilor, International Myopia Institute, SightGlass Vision and Thea. JSW reports grants from SightGlass Vision and Espansione and shares in Wolffsohn Research Limited.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
