Opioids for the palliation of symptoms in people with serious respiratory illness: a systematic review and meta-analysis
- PMID: 39384304
- PMCID: PMC11462312
- DOI: 10.1183/16000617.0265-2023
Opioids for the palliation of symptoms in people with serious respiratory illness: a systematic review and meta-analysis
Abstract
Background: People living with serious respiratory illness experience a high burden of distressing symptoms. Although opioids are prescribed for symptom management, they generate adverse events, and their benefits are unclear.
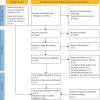
Methods: We examined the efficacy and safety of opioids for symptom management in people with serious respiratory illness. Embase, MEDLINE and the Cochrane Central Register of Controlled Trials were searched up to 11 July 2022. Reports of randomised controlled trials administering opioids to treat symptoms in people with serious respiratory illness were included. Key exclusion criteria included <80% of participants having a nonmalignant lung disease. Data were extracted regarding study characteristics, outcomes of breathlessness, cough, health-related quality of life (HRQoL) and adverse events. Treatment effects were pooled using a generic inverse variance model with random effects. Risk of bias was assessed using the Cochrane Risk of Bias tool version 1.
Results: Out of 17 included trials, six were laboratory-based exercise trials (n=70), 10 were home studies measuring breathlessness in daily life (n=788) and one (n=18) was conducted in both settings. Overall certainty of evidence was "very low" to "low". Opioids reduced breathlessness intensity during laboratory exercise testing (standardised mean difference (SMD) -0.37, 95% CI -0.67- -0.07), but not breathlessness measured in daily life (SMD -0.10, 95% CI -0.64-0.44). No effects on HRQoL (SMD -0.42, 95% CI -0.98-0.13) or cough (SMD -1.42, 95% CI -3.99-1.16) were detected. In at-home studies, opioids led to increased frequency of nausea/vomiting (OR 3.32, 95% CI 1.70-6.51), constipation (OR 3.08, 95% CI 1.69-5.61) and drowsiness (OR 1.37, 95% CI 1.01-1.86), with serious adverse events including hospitalisation and death identified.
Conclusions: Opioids improved exertional breathlessness in laboratory exercise studies, but did not improve breathlessness, cough or HRQoL measured in daily life at home. There were significant adverse events, which may outweigh any benefits.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: N.E. Smallwood reports grants from NHMRC, MRFF, Cancer Council Australia, Fisher & Paykel Healthcare (FPH), Windermere Foundation, Lung Foundation Australia, Lord Mayor's Foundation Melbourne and Bethlehem Griffiths Foundation, consulting fees from The Limbic and Orchard Consulting, lecture honoraria from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, FPH and Health Ed, travel support from Chiesi and Boehringer Ingelheim, leadership roles as Board Director and past state president of the Thoracic Society of Australia and New Zealand, Board Director of Victorian Doctors’ Program and co-chair of the guidelines committee for European Respiratory Society; and receipt of equipment from FPH, outside the submitted work. M. Wijsenbeek reports grants from The Netherlands Organisation for Health Research and Development, The Dutch Lung Foundation, The Dutch Pulmonary Fibrosis organization, Sarcoidosis.nl, Boehringer Ingelheim, Hoffman la Roche and AstraZeneca-Daiichi, consulting fees from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Galapagos, Galecto, GSK, Hoffman la Roche, Horizon therapeutics, Kinevant Sciences, Molecure, Nerre Therapeutics, Novartis, PureTech Health, Thyron, Trevi and Vicore, lecture honoraria from Boehringer Ingelheim, CSL Behring, Hoffman la Roche and Novartis, travel support from Boehringer Ingelheim, GSK, Hoffman la Roche and Galapagos, advisory board participation with Galapagos, and leadership roles as Chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, Member of the board of the Netherlands Respiratory Society, Member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and related disorders federation, Chair of the educational committee of the European Reference Network for rare Lung Diseases, and membership of the advisory board of the Dutch Lungfibrosis and Sarcoidosis patient associations, outside the submitted work. A-M. Russell reports consulting fees from Boehringer Ingelheim, and lecture honoraria from Boehringer Ingelheim, Hoffman La Roche and the Irish Lung Fibrosis Association, outside the submitted work. A.E. Holland is President of Thoracic Society of Australia and New Zealand, an unpaid position unrelated to the present work. All other authors have nothing to disclose.
Figures



Comment in
-
Evidence-based management of symptoms in serious respiratory illness: what is in our toolbox?Eur Respir Rev. 2024 Oct 30;33(174):240205. doi: 10.1183/16000617.0205-2024. Print 2024 Oct. Eur Respir Rev. 2024. PMID: 39477357 Free PMC article.
References
-
- Swetz KM, Shanafelt TD, Drozdowicz LB, et al. Symptom burden, quality of life, and attitudes toward palliative care in patients with pulmonary arterial hypertension: results from a cross-sectional patient survey. J Heart Lung Transplant 2012; 31: 1102–1108. doi: 10.1016/j.healun.2012.08.010 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
