Home use of mifepristone for medical abortion: a systematic review
- PMID: 39384382
- PMCID: PMC12322458
- DOI: 10.1136/bmjsrh-2024-202302
Home use of mifepristone for medical abortion: a systematic review
Abstract
Background: In many countries, persons seeking medical abortion with mifepristone followed by misoprostol can self-administer the second drug, misoprostol, at home, but self-administration of the first drug, mifepristone, is not allowed to the same extent.
Objectives: This systematic review aims to evaluate whether the efficacy, safety and women's satisfaction with abortion treatment are affected when mifepristone is self-administered at home instead of in a clinic.
Search strategy: A literature search covered CINAHL, Cochrane Library, Embase, Ovid MEDLINE and APA PsycInfo in October 2022.
Selection criteria: Eligible studies focused on persons undergoing medical abortion comparing home and in-clinic mifepristone intake. Outcomes included abortion effectiveness, compliance, acceptability, and practical consequences for women.
Data collection and analysis: Two reviewers independently assessed eligibility and risk of bias. Meta-analysis included similar studies while those differing in design were synthesised without meta-analysis.
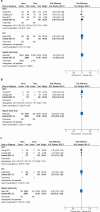
Results: Six studies (54 233 women) of medical abortions up to 10 weeks were included. One randomised controlled trial and one retrospective register study had moderate risk of bias, and four non-randomised clinical trials where women could choose the place for intake of mifepristone had serious risk of bias. There was no difference in abortion effectiveness (high confidence) or compliance (moderate confidence) between mifepristone administered at home or in-clinic. No differences in complications were detected between groups and most women who chose home administration of mifepristone expressed a preference for this approach.
Conclusions: Our systematic review demonstrates that the effectiveness of medical abortion is comparable regardless of mifepristone administration and intake, at home or in the clinic.
Keywords: Abortifacient Agents; Mifepristone; abortion.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: KGD has received honorarium for lectures or advice on abortion and contraception on ad hoc basis for Organon, Bayer, Gedeon Richter, Exelgyn, Mithra, Exeltis, Medincell, HRA Pharma, Cirqle, Natural cycles, Obseva, Norgene, and Ferring. IL has received compensation from Gedeon Richter and Exeltis for lectures in hormonal contraception and participation in an Advisory Board. JB has received honorariums for giving lectures in hormonal contraception for Organon, Campus Pharma, Exeltis and Gedeon Richter. SJ, AC, KM and EW have no conflicts of interest to declare.
Figures

Similar articles
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2004;(2):CD002855. doi: 10.1002/14651858.CD002855.pub3. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Nov 09;(11):CD002855. doi: 10.1002/14651858.CD002855.pub4. PMID: 15106180 Updated.
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2004;(1):CD002855. doi: 10.1002/14651858.CD002855.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2004;(2):CD002855. doi: 10.1002/14651858.CD002855.pub3. PMID: 14973995 Updated.
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2011 Nov 9;2011(11):CD002855. doi: 10.1002/14651858.CD002855.pub4. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2022 May 24;5:CD002855. doi: 10.1002/14651858.CD002855.pub5. PMID: 22071804 Free PMC article. Updated.
-
The use of telemedicine services for medical abortion.Cochrane Database Syst Rev. 2025 Jun 4;6(6):CD013764. doi: 10.1002/14651858.CD013764.pub2. Cochrane Database Syst Rev. 2025. PMID: 40464275 Free PMC article. Review.
-
Methods for managing miscarriage: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jun 1;6(6):CD012602. doi: 10.1002/14651858.CD012602.pub2. Cochrane Database Syst Rev. 2021. PMID: 34061352 Free PMC article.
Cited by
-
Perspectives on Abortion Services, the Pre-Abortion Visit, and Telemedicine Abortion: A Qualitative Study in Sweden.Perspect Sex Reprod Health. 2025 Mar;57(1):36-44. doi: 10.1111/psrh.12290. Epub 2025 Jan 12. Perspect Sex Reprod Health. 2025. PMID: 39800999 Free PMC article.
References
-
- WHO . Abortion Care Guideline. Geneva: World Health Organization (WHO); 2022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
