Attenuation of the neuropathogenic equine herpesvirus type 1 strain Ab4p in hamsters by a single amino acid mutation (D752N) in viral DNA polymerase ORF30
- PMID: 39384384
- PMCID: PMC11612248
- DOI: 10.1292/jvms.24-0338
Attenuation of the neuropathogenic equine herpesvirus type 1 strain Ab4p in hamsters by a single amino acid mutation (D752N) in viral DNA polymerase ORF30
Abstract
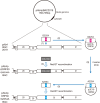
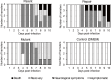
Equine herpesvirus type 1 (EHV-1) causes abortion, respiratory infection, and neurological diseases (equine herpesvirus myeloencephalopathy) in horses. A single nucleotide polymorphism (SNP) associated with a single amino acid in the DNA polymerase gene (ORF30, in which D752 is neuropathogenic and N752 is non-neuropathogenic) of EHV-1 has been associated with neuropathogenicity in horses. We constructed an EHV-1 Ab4p ORF30 N752 mutant and a repair virus to examine the effect of a D752N mutation on the neuropathogenicity of the virus in Syrian hamsters. The N752 mutation did not affect viral growth in cultured cells but it did attenuate the neuropathogenicity of Ab4p in the hamsters. The results suggest that D752N is involved in neuropathogenicity not only in horses but also in hamsters.
Keywords: DNA polymerase; ORF30; a single nucleotide polymorphism (SNP); equine herpesvirus; equine herpesvirus encephalopathy (EHM).
Conflict of interest statement
The authors have nothing to disclose.
Figures
References
-
- Frampton AR, Jr, Smith PM, Zhang Y, Grafton WD, Matsumura T, Osterrieder N, O’Callaghan DJ. 2004. Meningoencephalitis in mice infected with an equine herpesvirus 1 strain KyA recombinant expressing glycoprotein I and glycoprotein E. Virus Genes 29: 9–17. doi: 10.1023/B:VIRU.0000032785.19420.14 - DOI - PubMed

