Cellular senescence by loss of Men1 in osteoblasts is critical for age-related osteoporosis
- PMID: 39384404
- PMCID: PMC11464108
- DOI: 10.1111/acel.14254
Cellular senescence by loss of Men1 in osteoblasts is critical for age-related osteoporosis
Abstract
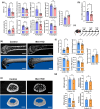
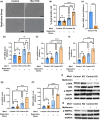
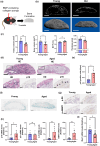
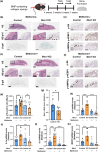
Recent evidence suggests an association between age-related osteoporosis and cellular senescence in the bone; however, the specific bone cells that play a critical role in age-related osteoporosis and the mechanism remain unknown. Results revealed that age-related osteoporosis is characterized by the loss of osteoblast Men1. Osteoblast-specific inducible knockout of Men1 caused structural changes in the mice bones, matching the phenotypes in patients with age-related osteoporosis. Histomorphometrically, Men1-knockout mice femurs decreased osteoblastic activity and increased osteoclastic activity, hallmarks of age-related osteoporosis. Loss of Men1 induces cellular senescence via mTORC1 activation and AMPK suppression, rescued by metformin treatment. In bone morphogenetic protein-indued bone model, loss of Men1 leads to accumulation of senescent cells and osteoporotic bone formation, which are ameliorated by metformin. Our results indicate that cellular senescence in osteoblasts plays a critical role in age-related osteoporosis and that osteoblast-specific inducible Men1-knockout mice offer a promising model for developing therapeutics for age-related osteoporosis.
Keywords: AMPK; Men1; cellular senescence; mTORC1; osteoporosis.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Anisimov, V. N. , Berstein, L. M. , Popovich, I. G. , Zabezhinski, M. A. , Egormin, P. A. , Piskunova, T. S. , Semenchenko, A. V. , Tyndyk, M. L. , Yurova, M. N. , Kovalenko, I. G. , & Poroshina, T. E. (2011). If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY), 3(2), 148–157. 10.18632/aging.100273 - DOI - PMC - PubMed
-
- Baker, D. J. , Childs, B. G. , Durik, M. , Wijers, M. E. , Sieben, C. J. , Zhong, J. , Saltness, R. A. , Jeganathan, K. B. , Verzosa, G. C. , Pezeshki, A. , Khazaie, K. , Miller, J. D. , & van Deursen, J. M. (2016). Naturally occurring p16Ink4a‐positive cells shorten healthy lifespan. Nature, 530(7589), 184–189. 10.1038/nature16932 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

