Phase II study of novel CXCR2 agonist and Plerixafor for rapid stem cell mobilization in patients with multiple myeloma
- PMID: 39384609
- PMCID: PMC11464886
- DOI: 10.1038/s41408-024-01152-1
Phase II study of novel CXCR2 agonist and Plerixafor for rapid stem cell mobilization in patients with multiple myeloma
Abstract
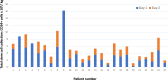
MGTA-145 or GROβT, a CXCR2 agonist, has shown promising activity for hematopoietic stem cell (HSC) mobilization with plerixafor in pre-clinical studies and healthy volunteers. Twenty-five patients with multiple myeloma enrolled in a phase 2 trial evaluating MGTA-145 and plerixafor for HSC mobilization (NCT04552743). Plerixafor was given subcutaneously followed 2 h later by MGTA-145 (0.03 mg/kg) intravenously with same day apheresis. Mobilization/apheresis could be repeated for a second day in patients who collected <6 ×106 CD34+ cells/kg. Lenalidomide and anti-CD38 antibody were part of induction therapy in 92% (n = 23) and 24% (n = 6) of patients, respectively. Median total HSC cell yield (CD34+ cells/kg × 106) was 5.0 (range: 1.1-16.2) and day 1 yield was 3.4 (range: 0.3-16.2). 88% (n = 22) of patients met the primary endpoint of collecting 2 ×106 CD34+ cells/kg in ≤ two days, 68% (n = 17) in one day. Secondary endpoints of collecting 4 and 6 × 106 CD34+ cells/kg in ≤ two days were met in 68% (n = 17) and 40% (n = 10) patients. Grade 1 or 2 adverse events (AE) were seen in 60% of patients, the most common AE being grade 1 pain, usually self-limited. All 19 patients who underwent transplant with MGTA-145 and plerixafor mobilized HSCs engrafted successfully, with durable engraftment at day 100. 74% (17 of 23) of grafts with this regimen were minimal residual disease negative by next generation flow cytometry. Graft composition for HSCs and immune cells were similar to a contemporaneous cohort mobilized with G-CSF and plerixafor.
© 2024. The Author(s).
Conflict of interest statement
SS (Consultancy: Magenta Therapeutics, now known as Dianthus therapeutics).
Figures


References
-
- DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–6. - PubMed
-
- Giralt S, Costa L, Schriber J, DiPersio J, Maziarz R, McCarty J, et al. Optimizing Autologous Stem Cell Mobilization Strategies to Improve Patient Outcomes: Consensus Guidelines and Recommendations. Biol Blood Marrow Transplant. 2014;20:295–308. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

