AXIN1 boosts antiviral response through IRF3 stabilization and induced phase separation
- PMID: 39384753
- PMCID: PMC11464762
- DOI: 10.1038/s41392-024-01978-y
AXIN1 boosts antiviral response through IRF3 stabilization and induced phase separation
Abstract
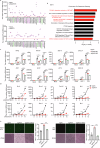
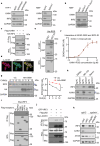
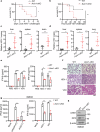
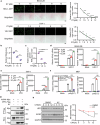
Axis inhibition protein 1 (AXIN1), a scaffold protein interacting with various critical molecules, plays a vital role in determining cell fate. However, its impact on the antiviral innate immune response remains largely unknown. Here, we identify that AXIN1 acts as an effective regulator of antiviral innate immunity against both DNA and RNA virus infections. In the resting state, AXIN1 maintains the stability of the transcription factor interferon regulatory factor 3 (IRF3) by preventing p62-mediated autophagic degradation of IRF3. This is achieved by recruiting ubiquitin-specific peptidase 35 (USP35), which removes lysine (K) 48-linked ubiquitination at IRF3 K366. Upon virus infection, AXIN1 undergoes a phase separation triggered by phosphorylated TANK-binding kinase 1 (TBK1). This leads to increased phosphorylation of IRF3 and a boost in IFN-I production. Moreover, KYA1797K, a small molecule that binds to the AXIN1 RGS domain, enhances the AXIN1-IRF3 interaction and promotes the elimination of various highly pathogenic viruses. Clinically, patients with HBV-associated hepatocellular carcinoma (HCC) who show reduced AXIN1 expression in pericarcinoma tissues have low overall and disease-free survival rates, as well as higher HBV levels in their blood. Overall, our findings reveal how AXIN1 regulates IRF3 signaling and phase separation-mediated antiviral immune responses, underscoring the potential of the AXIN1 agonist KYA1797K as an effective antiviral agent.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Arzumanyan, A., Reis, H. M. & Feitelson, M. A. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer13, 123–135, (2013). - PubMed
-
- Chen, Q., Sun, L. & Chen, Z. J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol17, 1142–1149, (2016). - PubMed
-
- Zhang, X., Bai, X. C. & Chen, Z. J. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity53, 43–53 (2020). - PubMed
-
- Shulman, Z. & Stern-Ginossar, N. The RNA modification N(6)-methyladenosine as a novel regulator of the immune system. Nat Immunol21, 501–512 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

