β-synuclein regulates the phase transitions and amyloid conversion of α-synuclein
- PMID: 39384788
- PMCID: PMC11464764
- DOI: 10.1038/s41467-024-53086-8
β-synuclein regulates the phase transitions and amyloid conversion of α-synuclein
Abstract
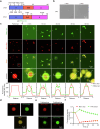
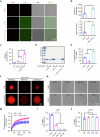
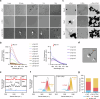
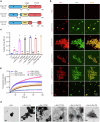
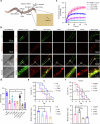
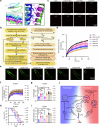
Parkinson's disease (PD) and Dementia with Lewy Bodies (DLB) are neurodegenerative disorders characterized by the accumulation of α-synuclein aggregates. α-synuclein forms droplets via liquid-liquid phase separation (LLPS), followed by liquid-solid phase separation (LSPS) to form amyloids, how this process is physiologically-regulated remains unclear. β-synuclein colocalizes with α-synuclein in presynaptic terminals. Here, we report that β-synuclein partitions into α-synuclein condensates promotes the LLPS, and slows down LSPS of α-synuclein, while disease-associated β-synuclein mutations lose these capacities. Exogenous β-synuclein improves the movement defects and prolongs the lifespan of an α-synuclein-expressing NL5901 Caenorhabditis elegans strain, while disease-associated β-synuclein mutants aggravate the symptoms. Decapeptides targeted at the α-/β-synuclein interaction sites are rationally designed, which suppress the LSPS of α-synuclein, rescue the movement defects, and prolong the lifespan of C. elegans NL5901. Together, we unveil a Yin-Yang balance between α- and β-synuclein underlying the normal and disease states of PD and DLB with therapeutical potentials.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
β-synuclein blocks α-synuclein condensate fusion to disrupt the maturation of phase separation.Cell Rep. 2025 Jun 24;44(6):115761. doi: 10.1016/j.celrep.2025.115761. Epub 2025 May 28. Cell Rep. 2025. PMID: 40440171
-
Distinct Effects of Familial Parkinson's Disease-Associated Mutations on α-Synuclein Phase Separation and Amyloid Aggregation.Biomolecules. 2023 Apr 23;13(5):726. doi: 10.3390/biom13050726. Biomolecules. 2023. PMID: 37238596 Free PMC article.
-
Observation of an α-synuclein liquid droplet state and its maturation into Lewy body-like assemblies.J Mol Cell Biol. 2021 Aug 4;13(4):282-294. doi: 10.1093/jmcb/mjaa075. J Mol Cell Biol. 2021. PMID: 33386842 Free PMC article.
-
Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
-
[Clinical and pathological study on early diagnosis of Parkinson's disease and dementia with Lewy bodies].Rinsho Shinkeigaku. 2008 Jan;48(1):11-24. doi: 10.5692/clinicalneurol.48.11. Rinsho Shinkeigaku. 2008. PMID: 18386627 Review. Japanese.
Cited by
-
Trends in the research and development of peptide drug conjugates: artificial intelligence aided design.Front Pharmacol. 2025 Feb 27;16:1553853. doi: 10.3389/fphar.2025.1553853. eCollection 2025. Front Pharmacol. 2025. PMID: 40083376 Free PMC article. Review.
-
Ferroptosis and Iron Homeostasis: Molecular Mechanisms and Neurodegenerative Disease Implications.Antioxidants (Basel). 2025 Apr 28;14(5):527. doi: 10.3390/antiox14050527. Antioxidants (Basel). 2025. PMID: 40427409 Free PMC article. Review.
-
Angiopoietin-like protein 8 directs DNA damage responses towards apoptosis by stabilizing PARP1-DNA condensates.Cell Death Differ. 2025 Apr;32(4):672-688. doi: 10.1038/s41418-024-01422-2. Epub 2024 Nov 27. Cell Death Differ. 2025. PMID: 39592710
-
Studying the mechanisms of neurodegeneration: C. elegans advantages and opportunities.Front Cell Neurosci. 2025 Mar 26;19:1559151. doi: 10.3389/fncel.2025.1559151. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40207239 Free PMC article. Review.
-
Soluble and Insoluble Lysates from the Human A53T Mutant α-Synuclein Transgenic Mouse Model Induces α-Synucleinopathy Independent of Injection Site.Int J Mol Sci. 2025 Jun 28;26(13):6254. doi: 10.3390/ijms26136254. Int J Mol Sci. 2025. PMID: 40650031 Free PMC article.
References
-
- Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science357, eaaf4382 (2017). - PubMed
-
- Chakraborty, P. & Zweckstetter, M. Role of aberrant phase separation in pathological protein aggregation. Curr. Opin. Struct. Biol.82, 102678 (2023). - PubMed
-
- Castillo-Barnes, D. et al. Nonlinear weighting ensemble learning model to diagnose Parkinson’s disease using multimodal data. Int J. Neural Syst.33, 2350041 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

