Tumour-informed liquid biopsies to monitor advanced melanoma patients under immune checkpoint inhibition
- PMID: 39384805
- PMCID: PMC11464631
- DOI: 10.1038/s41467-024-52923-0
Tumour-informed liquid biopsies to monitor advanced melanoma patients under immune checkpoint inhibition
Abstract
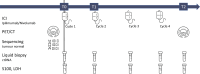
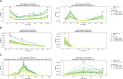
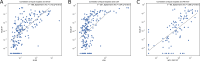
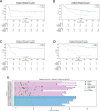
Immune checkpoint inhibitors (ICI) have significantly improved overall survival in melanoma patients. However, 60% experience severe adverse events and early response markers are lacking. Circulating tumour DNA (ctDNA) is a promising biomarker for treatment-response and recurrence detection. The prospective PET/LIT study included 104 patients with palliative combined or adjuvant ICI. Tumour-informed sequencing panels to monitor 30 patient-specific variants were designed and 321 liquid biopsies of 87 patients sequenced. Mean sequencing depth after deduplication using UMIs was 6000x and the error rate of UMI-corrected reads was 2.47×10-4. Variant allele fractions correlated with PET/CT MTV (rho=0.69), S100 (rho=0.72), and LDH (rho=0.54). A decrease of allele fractions between T1 and T2 was associated with improved PFS and OS in the palliative cohort (p = 0.008 and p < 0.001). ctDNA was detected in 76.9% of adjuvant patients with relapse (n = 10/13), while all patients without progression (n = 9) remained ctDNA negative. Tumour-informed liquid biopsies are a reliable tool for monitoring treatment response and early relapse in melanoma patients with ICI.
© 2024. The Author(s).
Conflict of interest statement
A.F. reports honoraria for presentations for BMS, MSD, Novartis, Pierre-Fabre, Delcath and Immunocore; travel support and congress participation support from BMS, Pierre-Fabre, Novartis, MSD; Advisory Boards from MSD, BMS, Novartis, Pierre-Fabre, Immunocore and research funding from BMS Stiftung Immunonkologie. C.S. and S.O. report research funding from BMS Stiftung Immunonkologie as well as institutional grants and payment for conference presentations from Illumina Inc. and Oxford Nanopore Technologies outside the submitted work. The remaining authors have no conflict of interests to disclose.
Figures
References
-
- Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med.372, 320–330 (2015). - PubMed
-
- Ribas, A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA315, 1600–1609 (2016). - PubMed
-
- Hodi, F. S. et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol.19, 1480–1492 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

