Disordered regions of human eIF4B orchestrate a dynamic self-association landscape
- PMID: 39384813
- PMCID: PMC11464913
- DOI: 10.1038/s41467-024-53136-1
Disordered regions of human eIF4B orchestrate a dynamic self-association landscape
Abstract
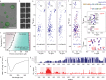
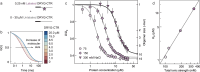
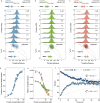
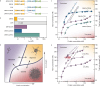
Eukaryotic translation initiation factor eIF4B is required for efficient cap-dependent translation, it is overexpressed in cancer cells, and may influence stress granule formation. Due to the high degree of intrinsic disorder, eIF4B is rarely observed in cryo-EM structures of translation complexes and only ever by its single structured RNA recognition motif domain, leaving the molecular details of its large intrinsically disordered region (IDR) unknown. By integrating experiments and simulations we demonstrate that eIF4B IDR orchestrates and fine-tunes an intricate transition from monomers to a condensed phase, in which large-size dynamic oligomers form before mesoscopic phase separation. Single-molecule spectroscopy combined with molecular simulations enabled us to characterize the conformational ensembles and underlying intra- and intermolecular dynamics across the oligomerization transition. The observed sensitivity to ionic strength and molecular crowding in the self-association landscape suggests potential regulation of eIF4B nanoscopic and mesoscopic behaviors such as driven by protein modifications, binding partners or changes to the cellular environment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

