Antifibrotic effect of disulfiram on bleomycin-induced lung fibrosis in mice and its impact on macrophage infiltration
- PMID: 39384840
- PMCID: PMC11464646
- DOI: 10.1038/s41598-024-71770-z
Antifibrotic effect of disulfiram on bleomycin-induced lung fibrosis in mice and its impact on macrophage infiltration
Abstract
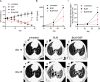
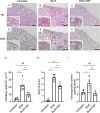
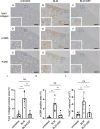
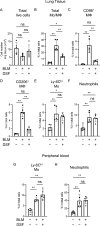
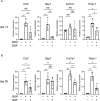
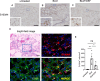
The accumulation of monocyte-derived macrophages in the lung tissue during inflammation is important for the pathogenesis of fibrotic lung disease. Deficiencies in chemokine receptors CCR2 and CCR5 and their ligands, which mediate monocyte/macrophage migration, ameliorate bleomycin (BLM)-induced lung fibrosis. Disulfiram (DSF), which is used to treat alcoholism because of its aldehyde dehydrogenase (ALDH)-inhibiting effect, inhibits monocyte/macrophage migration by inhibiting FROUNT, an intracellular regulator of CCR2/CCR5 signalling. Here, we investigated the antifibrotic effect of oral DSF administration in a mouse model of BLM-induced lung fibrosis, focusing on macrophage response and fibrosis progression. The direct inhibitory activity of DSF on monocyte migration was measured using the Boyden chamber assay and compared with that of DSF-related inhibitors with different FROUNT-inhibition activities. Quantitative PCR was used to determine the expression of fibrosis-promoting genes in the lung tissue. DSF significantly suppressed macrophage infiltration into lung tissues and attenuated BLM-induced lung fibrosis. DSF and its metabolites, diethyldithiocarbamate (DDC) and copper diethyldithiocarbamate (Cu(DDC)2), inhibited monocyte migration toward the culture supernatant of primary mouse lung cells mainly comprising CCL2, whereas cyanamide, another ALDH inhibitor, did not. DSF, with higher inhibitory activity against FROUNT than DDC and Cu(DDC)2, inhibited monocyte migration most strongly. In BLM-induced fibrotic lung tissues, profibrotic factors were highly expressed but were reduced by DSF treatment. These results suggest DSF inhibits macrophage infiltration, which might be attributed to its inhibitory effect on FROUNT, and attenuates BLM-induced lung fibrosis. In addition, multiplex immunofluorescence imaging revealed reduced infiltration of S100A4+ macrophages into the lungs in DSF-treated mice and high expression of FROUNT in S100A4+ macrophages in idiopathic pulmonary fibrosis (IPF). These findings underscore the potential of macrophage-targeted therapy with DSF as a promising drug repositioning approach for treating fibrotic lung diseases, including IPF.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

