Multicellular ovarian cancer spheroids: novel 3D model to mimic tumour complexity
- PMID: 39384844
- PMCID: PMC11464915
- DOI: 10.1038/s41598-024-73680-6
Multicellular ovarian cancer spheroids: novel 3D model to mimic tumour complexity
Abstract
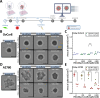
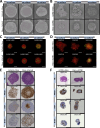
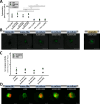
In vitro, spheroid models have become well established in cancer research because they can better mimic certain characteristics of in vivo tumours. However, interaction with the tumour microenvironment, such as cancer-associated fibroblasts, plays a key role in tumour progression. We initially focused on the interaction of tumour cells with fibroblasts. To model this interaction, we developed a spheroid model of ovarian cancer and fibroblasts. To this end, ovarian cancer cell lines and ex vivo primary cells were simultaneously and sequentially seeded with fibroblasts in a scaffold-free system at different ratios and subsequently characterized with respect to changes in morphology, proliferation, and viability. We demonstrated that co-cultures are able to form by far more compact spheroids, especially in cells that form aggregates in mono-culture. In addition, the co-cultures were able to increase proliferation and sensitivity to cisplatin. Simultaneous seeding led fibroblasts invade the core in both cell lines and primary cells. These results show differences in formation, firmness, and size between co-culture and mono-culture. Our model is designed to better represent and characterize the mutual influencing factors of fibroblasts and tumour cells. Fibroblast-supplemented multicellular spheroids are a valuable tool for tumour microenvironment interaction and new drug discovery.
Keywords: Co-culture model; Ovarian cancer and fibroblast; Spheroids; Tumour microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

