A promiscuous mechanism to phase separate eukaryotic carbon fixation in the green lineage
- PMID: 39384944
- PMCID: PMC11570498
- DOI: 10.1038/s41477-024-01812-x
A promiscuous mechanism to phase separate eukaryotic carbon fixation in the green lineage
Abstract
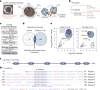
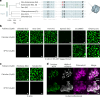
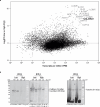
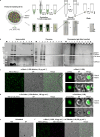
CO2 fixation is commonly limited by inefficiency of the CO2-fixing enzyme Rubisco. Eukaryotic algae concentrate and fix CO2 in phase-separated condensates called pyrenoids, which complete up to one-third of global CO2 fixation. Condensation of Rubisco in pyrenoids is dependent on interaction with disordered linker proteins that show little conservation between species. We developed a sequence-independent bioinformatic pipeline to identify linker proteins in green algae. We report the linker from Chlorella and demonstrate that it binds a conserved site on the Rubisco large subunit. We show that the Chlorella linker phase separates Chlamydomonas Rubisco and that despite their separation by ~800 million years of evolution, the Chlorella linker can support the formation of a functional pyrenoid in Chlamydomonas. This cross-species reactivity extends to plants, with the Chlorella linker able to drive condensation of some native plant Rubiscos in vitro and in planta. Our results represent an exciting frontier for pyrenoid engineering in plants, which is modelled to increase crop yields.
© 2024. The Author(s).
Conflict of interest statement
Figures









Comment in
-
Algal pyrenoid protein can condense plant Rubiscos: a step towards boosting carbon fixation in crops.Nat Plants. 2024 Nov;10(11):1625-1626. doi: 10.1038/s41477-024-01813-w. Nat Plants. 2024. PMID: 39384946 No abstract available.
References
-
- Field, C. B., Behrenfeld, M. J., Randerson, J. T. & Falkowski, P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science281, 237–240 (1998). - PubMed
-
- Bar-Even, A. et al. The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry50, 4402–4410 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/Y000323/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- MR/T040742/1/MRC_/Medical Research Council/United Kingdom
- MR/T040742/1/RCUK | MRC | Medical Research Foundation
- BB/S015337/11/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- MR/T020679/1/RCUK | Medical Research Council (MRC)
- EP/W024063/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- WT_/Wellcome Trust/United Kingdom
- BB/T007222/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- 206161/Z/17/Z/Wellcome Trust (Wellcome)
- BB/M0111511a/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
LinkOut - more resources
Full Text Sources

