The interplay of mutagenesis and ecDNA shapes urothelial cancer evolution
- PMID: 39385020
- PMCID: PMC11541202
- DOI: 10.1038/s41586-024-07955-3
The interplay of mutagenesis and ecDNA shapes urothelial cancer evolution
Abstract
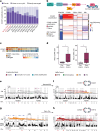
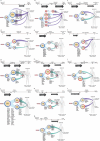
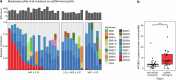
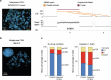
Advanced urothelial cancer is a frequently lethal disease characterized by marked genetic heterogeneity1. In this study, we investigated the evolution of genomic signatures caused by endogenous and external mutagenic processes and their interplay with complex structural variants (SVs). We superimposed mutational signatures and phylogenetic analyses of matched serial tumours from patients with urothelial cancer to define the evolutionary dynamics of these processes. We show that APOBEC3-induced mutations are clonal and early, whereas chemotherapy induces mutational bursts of hundreds of late subclonal mutations. Using a genome graph computational tool2, we observed frequent high copy-number circular amplicons characteristic of extrachromosomal DNA (ecDNA)-forming SVs. We characterized the distinct temporal patterns of APOBEC3-induced and chemotherapy-induced mutations within ecDNA-forming SVs, gaining new insights into the timing of these mutagenic processes relative to ecDNA biogenesis. We discovered that most CCND1 amplifications in urothelial cancer arise within circular ecDNA-forming SVs. ecDNA-forming SVs persisted and increased in complexity, incorporating additional DNA segments and contributing to the evolution of treatment resistance. Oxford Nanopore Technologies long-read whole-genome sequencing followed by de novo assembly mapped out CCND1 ecDNA structure. Experimental modelling of CCND1 ecDNA confirmed its role as a driver of treatment resistance. Our findings define fundamental mechanisms that drive urothelial cancer evolution and have important therapeutic implications.
© 2024. The Author(s).
Conflict of interest statement
B.M.F.: consulting or advisory role for QED therapeutics, Boston Gene, Astrin Biosciences Merck, Immunomedics/Gilead, QED therapeutics, Guardant and Janssen; patent royalties from Immunomedics/Gilead; research support from Eli Lilly; and honoraria from Urotoday. O.E.: stock and other ownership interests from Freenome, OneThree Biotech, Owkin and Volastra Therapeutics, and personal fees from Pionyr Immunotherapeutics and Champions Oncology. S.T.T.: consulting or advisory role for 4D Pharma, Abbvie, AIkido Pharma, Amgen, Astellas Pharma, Bayer, Blue Earth Diagnostics, Clarity Pharmaceuticals, Clovis Oncology, Convergent Therapeutics, Dendreon, Endocyte, Genentech, Genomic Health, Gilead Sciences, Immunomedics, Janssen, Karyopharm Therapeutics, Medivation, Myovant Sciences, Novartis, Pfizer, POINT Biopharma, QED Therapeutics, Sanofi, Seagen, Telix Pharmaceuticals and Tolmar; research funding from Abbvie, Amgen, Astellas Pharma, AstraZeneca, AVEO, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis Oncology, Dendreon, Endocyte, Exelixis, Genentech, Immunomedics, Inovio Pharmaceuticals, Janssen, Karyopharm Therapeutics, Lilly, Medivation, Merck, Millennium, Newlink Genetics, Novartis, POINT Biopharma, Progenics, Rexahn Pharmaceuticals, Sanofi and Stem CentRx; patents, royalties, other intellectual property or patent royalty from Immunomedics/Gilead; travel, accommodation and expenses from Amgen, Immunomedics and Sanofi; and uncompensated relationships from ATLAB Pharma and Phosplatin Therapeutics. D.M.N.: consulting or advisory role for AstraZeneca, and research funding from AstraZeneca, Boehringer Ingelheim, Clovis Oncology, Exelixis, Immumedics, Janssen, Novartis, Pfizer and Zenith Epigenetics. J.T.N.: consulting or advisory role for AIQ Solutions, and travel, accommodation or expenses from Digital Science Press. C.N.S.: consulting or advisory role for Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb/Medarex, Foundation Medicine, Genzyme, Immunomedics, IMPAC Medical Systems, Incyte, Medscape, Merck, MSD, Pfizer, Roche and UroToday. A.M.M.: consulting or advisory role for Eisai, Exelixis and Janssen. J.M.M.: research funding from Personal Genome Diagnostics, and travel, accommodation or expenses from Personal Genome Diagnostics. D.S.: research funding from Urogen Pharma, Cepheid, Anchiano and CryoLife. P.A.: stock and other ownership interests with Abyost Pharmaceuticals; consulting or advisory role for ArTara Therapeutics; research funding from Adaptive Biotechnologies, Janssen Oncology and natera; and patents, royalties or other intellectual property from intravesical imidazolium compounds and urine biomarkers patent application. D.D.N., W.F.H., T.R.C., H.G., J.M.S., M. Shah, Z.R.G., L.W., A.H., M. Sigouros, J. Manohar, J. Moyer, M.A.A., A. Semaan, S.C., F.M.R., D.W., M.O., R.R.S., W.L., H.L.V., A. Sboner, G.I. and N.R. declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

