Diverse anti-defence systems are encoded in the leading region of plasmids
- PMID: 39385022
- PMCID: PMC11541004
- DOI: 10.1038/s41586-024-07994-w
Diverse anti-defence systems are encoded in the leading region of plasmids
Abstract
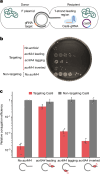
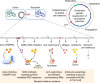
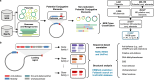
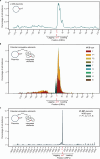
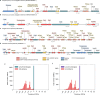
Plasmids are major drivers of gene mobilization by means of horizontal gene transfer and play a key role in spreading antimicrobial resistance among pathogens1,2. Despite various bacterial defence mechanisms such as CRISPR-Cas, restriction-modification systems and SOS-response genes that prevent the invasion of mobile genetic elements3, plasmids robustly transfer within bacterial populations through conjugation4,5. Here we show that the leading region of plasmids, the first to enter recipient cells, is a hotspot for an extensive repertoire of anti-defence systems, encoding anti-CRISPR, anti-restriction, anti-SOS and other counter-defence proteins. We further identified in the leading region a prevalence of promoters known to allow expression from single-stranded DNA6, potentially facilitating rapid protection against bacterial immunity during the early stages of plasmid establishment. We demonstrated experimentally the importance of anti-defence gene localization in the leading region for efficient conjugation. These results indicate that focusing on the leading region of plasmids could lead to the discovery of diverse anti-defence genes. Combined, our findings show a new facet of plasmid dissemination and provide theoretical foundations for developing efficient conjugative delivery systems for natural microbial communities.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med. Microbiol.303, 298–304 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

