Single-neuron representations of odours in the human brain
- PMID: 39385026
- PMCID: PMC11485236
- DOI: 10.1038/s41586-024-08016-5
Single-neuron representations of odours in the human brain
Abstract
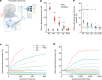
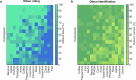
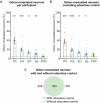
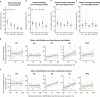
Olfaction is a fundamental sensory modality that guides animal and human behaviour1,2. However, the underlying neural processes of human olfaction are still poorly understood at the fundamental-that is, the single-neuron-level. Here we report recordings of single-neuron activity in the piriform cortex and medial temporal lobe in awake humans performing an odour rating and identification task. We identified odour-modulated neurons within the piriform cortex, amygdala, entorhinal cortex and hippocampus. In each of these regions, neuronal firing accurately encodes odour identity. Notably, repeated odour presentations reduce response firing rates, demonstrating central repetition suppression and habituation. Different medial temporal lobe regions have distinct roles in odour processing, with amygdala neurons encoding subjective odour valence, and hippocampal neurons predicting behavioural odour identification performance. Whereas piriform neurons preferably encode chemical odour identity, hippocampal activity reflects subjective odour perception. Critically, we identify that piriform cortex neurons reliably encode odour-related images, supporting a multimodal role of the human piriform cortex. We also observe marked cross-modal coding of both odours and images, especially in the amygdala and piriform cortex. Moreover, we identify neurons that respond to semantically coherent odour and image information, demonstrating conceptual coding schemes in olfaction. Our results bridge the long-standing gap between animal models and non-invasive human studies and advance our understanding of odour processing in the human brain by identifying neuronal odour-coding principles, regional functional differences and cross-modal integration.
© 2024. The Author(s).
Conflict of interest statement
K.O. is currently employed by dsm-firmenich. The company had no influence on the study design or interpretation of the results and did not provide any financial support. The other authors declare no competing interests.
Figures







References
-
- Wilson, D. A., Chapuis, J. & Sullivan, R. M. in Handbook of Olfaction and Gustation Ch. 10 (ed. Doty, R.) 209–224 (John Wiley & Sons, 2015); 10.1002/9781118971758.ch10.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

