Upregulation of keratin 15 is required for varicella-zoster virus replication in keratinocytes and is attenuated in the live attenuated vOka vaccine strain
- PMID: 39385182
- PMCID: PMC11465976
- DOI: 10.1186/s12985-024-02514-8
Upregulation of keratin 15 is required for varicella-zoster virus replication in keratinocytes and is attenuated in the live attenuated vOka vaccine strain
Abstract
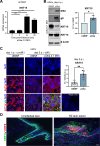
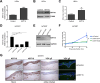
Varicella-zoster virus (VZV) is the etiological agent of chickenpox and shingles, diseases characterised by epidermal virus replication in skin and mucosa and the formation of blisters. We have previously shown that VZV infection has a profound effect on keratinocyte differentiation, altering the normal pattern of epidermal gene expression. In particular, VZV infection reduces expression of suprabasal keratins 1 and 10 and desmosomal proteins, disrupting epidermal structure to promote expression of a blistering phenotype. Here, we extend these findings to show that VZV infection upregulates the expression of keratin 15 (KRT15), a marker expressed by basal epidermal keratinocytes and hair follicles stem cells. We demonstrate that KRT15 is essential for VZV replication in the skin, since downregulation of KRT15 inhibits VZV replication in keratinocytes, while KRT15 exogenous overexpression supports viral replication. Importantly, our data show that VZV upregulation of KRT15 depends on the expression of the VZV immediate early gene ORF62. ORF62 is the only regulatory gene that is mutated in the live attenuated VZV vaccine and contains four of the five fixed mutations present in the VZV Oka vaccine. Our data indicate that the mutated vaccine ORF62 is not capable of upregulating KRT15, suggesting that this may contribute to the vaccine attenuation in skin. Taken together our data present a novel association between VZV and KRT15, which may open a new therapeutic window for a topical targeting of VZV replication in the skin via modulation of KRT15.
Keywords: IE62; KRT15; VZV; vOka vaccine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

