Autophagy controls neuronal differentiation by regulating the WNT-DVL signaling pathway
- PMID: 39385328
- PMCID: PMC12506719
- DOI: 10.1080/15548627.2024.2407707
Autophagy controls neuronal differentiation by regulating the WNT-DVL signaling pathway
Abstract
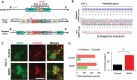
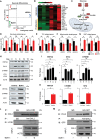
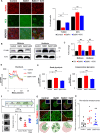
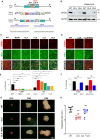
Macroautophagy/autophagy dysregulation is associated with various neurological diseases, including Vici syndrome. We aimed to determine the role of autophagy in early brain development. We generated neurons from human embryonic stem cells and developed a Vici syndrome model by introducing a loss-of-function mutation in the EPG5 gene. Autophagy-related genes were upregulated at the neuronal progenitor cell stage. Inhibition of autolysosome formation with bafilomycin A1 treatment at the neuronal progenitor cell stage delayed neuronal differentiation. Notably, WNT (Wnt family member) signaling may be part of the underlying mechanism, which is negatively regulated by autophagy-mediated DVL2 (disheveled segment polarity protein 2) degradation. Disruption of autolysosome formation may lead to failure in the downregulation of WNT signaling, delaying neuronal differentiation. EPG5 mutations disturbed autolysosome formation, subsequently inducing defects in progenitor cell differentiation and cortical layer generation in organoids. Disrupted autophagy leads to smaller organoids, recapitulating Vici syndrome-associated microcephaly, and validating the disease relevance of our study.Abbreviations: BafA1: bafilomycin A1; co-IP: co-immunoprecipitation; DVL2: dishevelled segment polarity protein 2; EPG5: ectopic P-granules 5 autophagy tethering factor; gRNA, guide RNA; hESC: human embryonic stem cells; KO: knockout; mDA, midbrain dopamine; NIM: neural induction media; NPC: neuronal progenitor cell; qPCR: quantitative polymerase chain reaction; UPS: ubiquitin-proteasome system; WNT: Wnt family member; WT: wild type.
Keywords: EPG5; Vici syndrome; WNT signal pathway; human embryonic stem cells; macroautophagy; neuronal differentiation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
