RNA sequencing reveals molecular mechanisms of endometriosis lesion development in mice
- PMID: 39385609
- PMCID: PMC11524442
- DOI: 10.1242/dmm.050566
RNA sequencing reveals molecular mechanisms of endometriosis lesion development in mice
Abstract
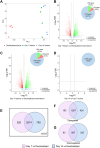
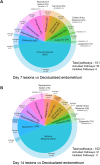
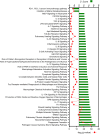
Understanding of molecular mechanisms contributing to the pathophysiology of endometriosis, and upstream drivers of lesion formation, remains limited. Using a C57Bl/6 mouse model in which decidualized endometrial tissue is injected subcutaneously in the abdomen of recipient mice, we generated a comprehensive profile of gene expression in decidualized endometrial tissue (n=4), and in endometriosis-like lesions at Day 7 (n=4) and Day 14 (n=4) of formation. High-throughput mRNA sequencing allowed identification of genes and pathways involved in the initiation and progression of endometriosis-like lesions. We observed distinct patterns of gene expression with substantial differences between the lesions and the decidualized endometrium that remained stable across the two lesion timepoints, and showed similarity to transcriptional changes implicated in human endometriosis lesion formation. Pathway enrichment analysis revealed several immune and inflammatory response-associated canonical pathways, multiple potential upstream regulators, and involvement of genes not previously implicated in endometriosis pathogenesis, including IRF2BP2 and ZBTB10, suggesting novel roles in disease progression. Collectively, the provided data will be a useful resource to inform research on the molecular mechanisms contributing to endometriosis-like lesion development in this mouse model.
Keywords: Endometriosis; Immune regulation; Mouse model; RNA Sequencing.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Acién, P., Quereda, F., Campos, A., Gomez-Torres, M.-J., Velasco, I. and Gutierrez, M. (2002). Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: a randomized clinical trial. Fertil. Steril. 78, 705-711. 10.1016/S0015-0282(02)03330-7 - DOI - PubMed
-
- Ali, A. F. M., Fateen, B., Ezzet, A., Badawy, H., Ramadan, A. and El-Tobge, A. (2000). Laparoscopic intraperitoneal injection of human interferon-α2b in the treatment of pelvic endometriosis: a new modality. Obstet. Gynecol. 95 4, Suppl. 1, S47-S48. 10.1016/S0029-7844(00)00684-0 - DOI
-
- Anastasi, E., Scaramuzzino, S., Viscardi, M. F., Viggiani, V., Piccioni, M. G., Cacciamani, L., Merlino, L., Angeloni, A., Muzii, L. and Porpora, M. G. (2023). Efficacy of N-acetylcysteine on endometriosis-related pain, size reduction of ovarian endometriomas, and fertility outcomes. Int. J. Environ. Res. Public Health 20, 4686. 10.3390/ijerph20064686 - DOI - PMC - PubMed
-
- Bacci, M., Capobianco, A., Monno, A., Cottone, L., Di Puppo, F., Camisa, B., Mariani, M., Brignole, C., Ponzoni, M., Ferrari, S.et al. (2009). Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 175, 547-556. 10.2353/ajpath.2009.081011 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

